Defects in Lyophilized Product: A Complete Easy Guide for All Ages Freeze-drying, or lyophilization, is a critical process used to preserve sensitive medicines, vaccines, and biological materials. However, during this process, some defects can occur that can affect the quality, safety, and effectiveness of the final product.
Understanding these defects helps improve quality control and ensure safety in pharmaceutical manufacturing. To learn more about the freeze-drying process, you can read this detailed article: Freeze-Drying Process Parameters.
Lyophilized products (those that have gone through freeze-drying) are usually found in glass vials sealed with rubber stoppers and aluminum caps. These products should appear as clean, dry, and uniform cakes. Any visible problems—like broken vials, particles, or color changes—are known as defects. These need to be carefully inspected and reported. For a complete checklist, explore this Freeze Dryer Batch Inspection Guide.

Key Takeaway
- Lyophilized product defects affect safety and effectiveness.
- Defects can be visual (like cracks or particles) or physical (like wrong dose or improper cake).
- Regular inspection and good manufacturing practices help avoid these issues.
What is a defect?
A defect is anything that goes wrong with the lyophilized product, vial, or packaging. It may include broken glass, improper cake shape, particles, or dose errors. For an in-depth overview, refer to Defect Classification in Lyophilized Products.
Types of Defects in Lyophilized Products
- Critical Defects—High risk, may harm the user.
- Major Defects—Significant, but may not directly harm.
- Minor Defects—Small issues that affect appearance or presentation.
Classification of Defects in Lyophilized Products
1. Critical Defects in Lyophilized Product
Critical defects are the most dangerous and unacceptable issues in lyophilized vials. These defects pose a direct risk to patient health and include broken or cracked vials, missing rubber stoppers, visible glass particles, cake melting or shrinkage, or liquid presence in the vial after drying. Products with critical defects must never be used. You can read more in this guide: How to Identify Critical Defects in Lyophilized Pharmaceuticals.

- Broken Vial: Completely shattered vial.
- Cracked Vial: Small cracks visible inside or outside the glass.
- Missing Stopper: No rubber stopper to seal the vial.
- Glass Particle: Visible broken glass inside the vial. (More on this)
- Color Variation: Product looks different in color than specified.
- Melt Back: The dried cake has shrunk. (Understand meltback)
- Shrinkage: The cake has visibly collapsed or shrunk.
- Liquid in Vial: Indicates improper drying or early stoppering.
- Exterior Spots/Powder: Dangerous for cytotoxic or hormone products.
Learn more about proper stoppering systems here: Freeze-Drying Process and Stoppering System.
2. Major Defects in Lyophilized Product
Major defects are serious issues in lyophilized vials that can affect product quality and patient safety but may not cause immediate harm. These defects include chipped glass, black or white particles, fiber contamination, dose variation (too much or too little powder), or vials with missing or incorrect coding. Major defects must be removed from the batch before product release. Explore best practices here: Lyophilization Validation Guide

- Chipped Glass: A small part is missing from the vial.
- Black/White Fibers: Long fiber particles visible in the cake.
- Black/White/Shining Particles: Tiny visible particles. (See guide.)
- Foreign Matter: Any unexpected particle in the vial.
- Dose on Stopper: Powder trapped between stopper and vial.
- High Dose: More powder than required.
- Low Dose: Less powder than required. (Dose variation explained)
- No Coding on Seal: Missing or unreadable code on the cap.
Read about dose variation issues in this article: Lyophilization Validation Best Practices.
3. Minor Defects in Lyophilized Product
Minor defects are small imperfections that usually do not affect the safety or effectiveness of the product. Examples include air bubbles in the glass, small scratches, slight staining, improper cake shape, or slightly dented seals. While not dangerous, these defects may still impact the visual appearance and quality standards.

- Air Bubbles: Small bubbles seen inside the glass.
- Black Particle in Glass: Embedded during glass formation.
- Deformed Vials: Vials that don’t match the standard shape.
- Scratches on Glass: Lines or marks on the surface.
- Stains: Black or brown stains on the vial body.
- Improper Cake: The shape of the dried cake is irregular. (More on appearance)
Sealing Defects in Lyophilized Product
Sealing issues refer to problems with the aluminum cap or flip-off part of the vial. Examples include dents, loose or missing flip-offs, irregular seal crimping, or poor coding. These issues can affect the vial’s ability to stay sterile and secure, which is important for preserving the medicine inside.
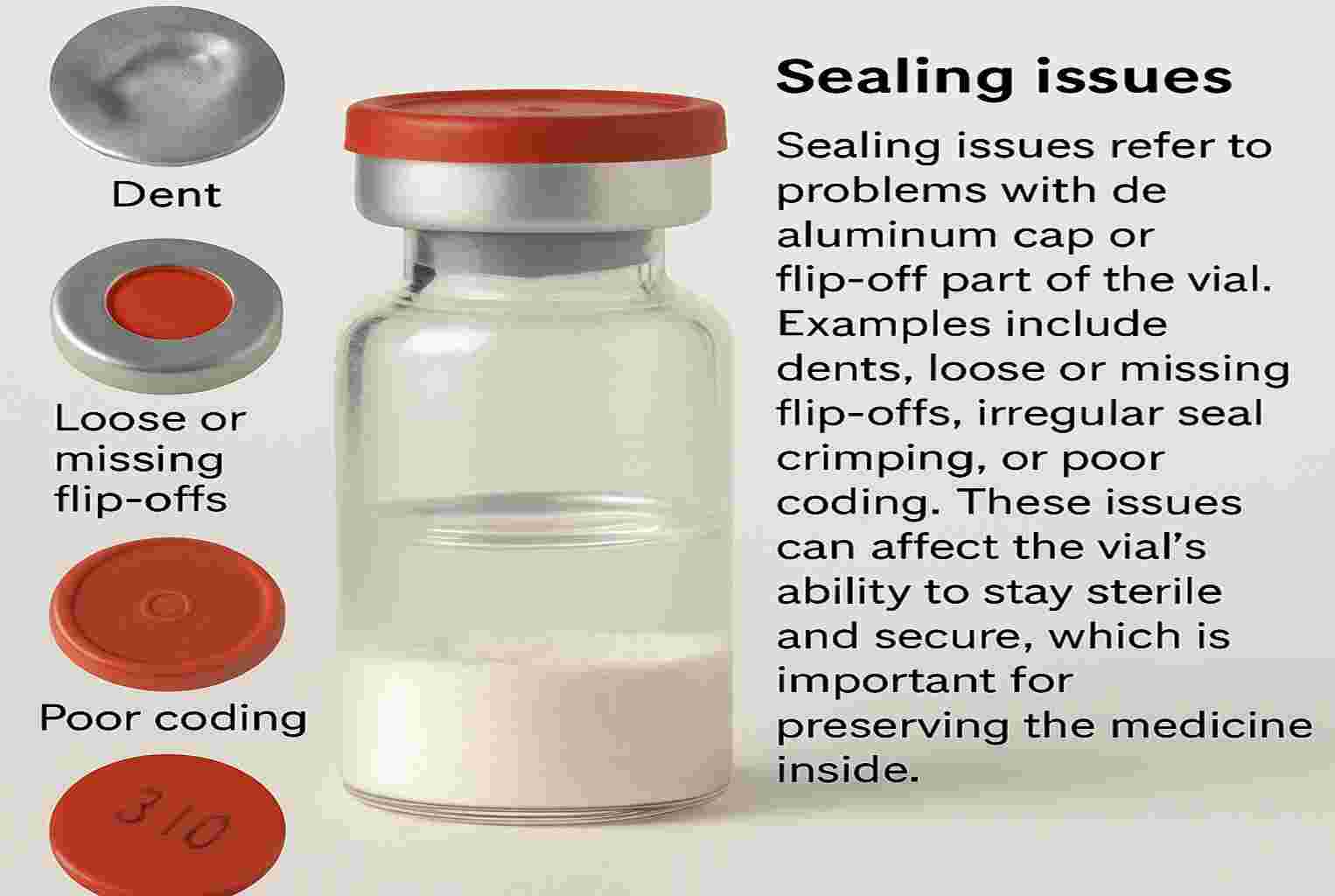
- Dots on Seal: Unwanted colored spots.
- Dented Seal: Visible dents.
- Improper Coding: Blurred or missing.
- Improper Crimping: Loose aluminum cap.
- Wavy Appearance: Irregular edges of the seal.
- Loose Flip-off: Loose plastic flip-off cap.
- Missing Flip-off: Completely missing cap.
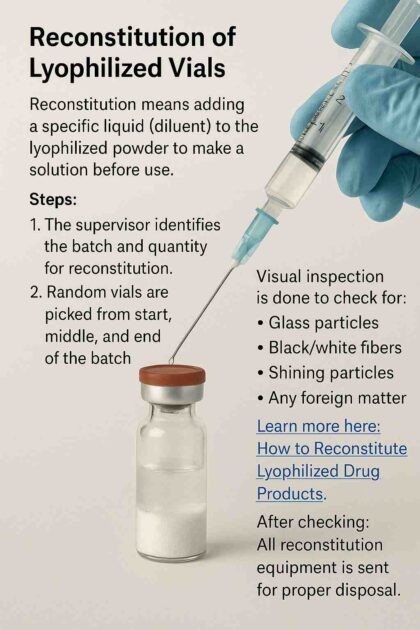
Reconstitution of Lyophilized Vials
Reconstitution means adding a specific liquid (diluent) to the lyophilized powder to make a solution before use.
Steps:
- The supervisor identifies the batch and quantity for reconstitution.
- Random vials are picked from the start, middle, and end of the batch.
- Use a clean syringe with a 0.2-micron filter to add diluent.
- Visual inspection is done to check for:
-
- Glass particles
- Black/white fibers
- Shining particles
- Any foreign matter
Learn more here: How to Reconstitute Lyophilized Drug Products.
After checking, all reconstitution equipment is sent for proper disposal.
Conclusion
Knowing about the possible defects in lyophilized products helps to ensure better manufacturing, safer medicines, and efficient product storage. For pharmaceutical industries, following inspection and safety protocols is essential to maintain high standards. To learn more about freeze dryer qualification, read Lyophilizer Qualification Guidelines. For more expert insights and troubleshooting tips, explore:
- Lyophilization Troubleshooting Guide
- Freeze Dryer Performance Testing Methodology
- Lyophilized Drug Stability
FAQs on Defects in Lyophilized Products
What is a lyophilized product?
A lyophilized product is a freeze-dried item, usually a medicine or vaccine, that has had its water removed to keep it stable for long-term storage.
Why do defects happen in lyophilized products?
Defects can happen due to problems in the freeze-drying process, packaging issues, or contamination. These affect the quality and safety of the product. Learn more in this comprehensive guide.
What are critical defects in lyophilized vials?
Critical defects include broken vials, cracks, missing stoppers, glass particles, and major cake issues like melt back or liquid inside the vial. These can be dangerous for users. See common causes.
What is meant by ‘melt back’ in lyophilized cake?
Meltback means the cake has shrunk or collapsed after drying, which shows that the freeze-drying process didn’t work properly. (Meltback explained)
How are major defects different from critical defects?
Major defects are serious but not immediately dangerous. Examples are chipped vials, fiber or black particles, or dose variation. See classification guide.
What is dose variation in lyophilized vials?
Dose variation means the powder inside the vial is either too much (high dose) or too little (low dose), which can affect how the medicine works. (Read more)
What is the importance of seal quality in lyophilized products?
The seal keeps the product safe and protected. Issues like dented seals, improper coding, or loose flip-offs are considered minor defects but still need attention. (Read sealing guide.)
What happens during the reconstitution of a lyophilized product?
Reconstitution is when a liquid (called a diluent) is added to the dried powder to turn it back into a usable solution. This is done before the medicine is given to the patient.
How are defects checked during reconstitution?
A clean syringe with a filter is used to add diluent. Then the solution is inspected for particles, fibers, and other defects under special lighting. (Learn procedure) Where can I learn more about freeze-drying and troubleshooting?
You can read expert articles like
👉 Freeze Dryer Troubleshooting Guide
👉 Lyophilizer Qualification Guidelines
👉 How to Reconstitute Lyophilized Drug Products