Lyophilizer Qualification Guidelines” article provides an in-depth exploration of lyophilization, also known as freeze-drying, a critical process for enhancing the stability and shelf-life of pharmaceutical products. It covers the three main stages—freezing, primary drying, and secondary drying—and examines the advantages and challenges of this process.
Additionally, the guide outlines the essential steps involved in lyophilization, from formulation preparation to product filling, environmental controls, and cycle validation. Special emphasis is placed on lyophilizer qualification, including equipment sterilization, leak detection, and preventive maintenance. With practical recommendations and detailed insights, the article serves as a comprehensive resource for professionals in pharmaceutical manufacturing and quality assurance
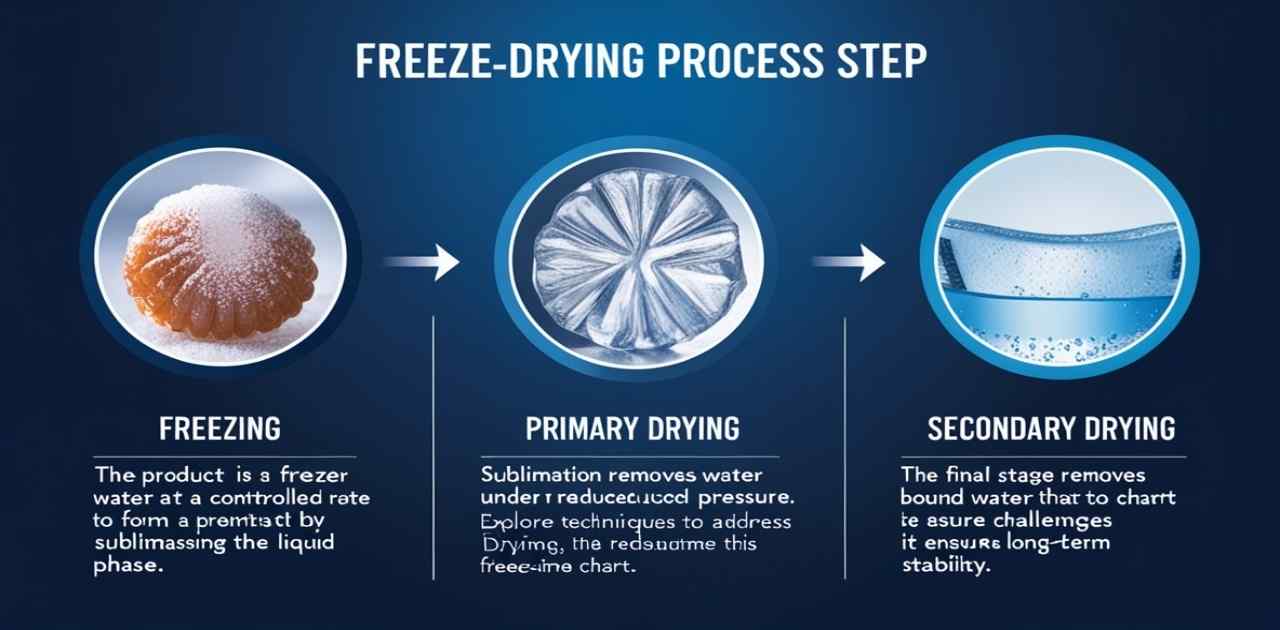
- Freeze-drying involves removing water from a product by sublimation, bypassing the liquid phase.
- The process includes three stages:
- Freezing: The product is frozen at a controlled rate to form ice crystals. Understanding the essential principles of freezing is crucial for success.
- Primary Drying: Sublimation removes water under reduced pressure. Explore techniques to address challenges in this freeze-drying time chart.
- Secondary Drying: The final stage removes bound water, ensuring long-term stability.
Advantages of Lyophilization
- Simplifies aseptic handling by processing liquids.
- Enhances stability of dry powders.
- Removes water without applying excessive heat to the product.
- Provides better stability in a dry state.
- Ensures rapid and easy dissolution upon reconstitution.
Disadvantages of Lyophilization
- Requires increased handling and processing time.
- Demands sterile diluents during reconstitution.
- Involves costly and complex equipment.
Steps in the Lyophilization Process
- Dissolve drugs and excipients in a suitable solvent, typically water for injection (WFI).
- Sterilize the bulk solution using a 0.2-micron bacteria-retentive filter.
- Fill sterile containers with the solution and partially stopper them under aseptic conditions.
- Transport partially stoppered containers to the lyophilizer in aseptic environments.
- Freeze the solution in the freeze-drying chamber or pre-freeze it elsewhere.
- Apply vacuum and heat the shelves to evaporate water from the frozen state.
- Completely stopper the vials using hydraulic or screw rod mechanisms.
Applications of Lyophilized Products
- Commonly used for anti-infectives, biotechnology-derived products, and in-vitro diagnostics.
- Issues like potency, sterility, and stability require careful management.
- Applications in Pharmaceuticals and Food: Lyophilization is invaluable in producing injectable drugs, vaccines, and food products. For insights into biopharmaceutical lyophilization trends, visit our market overview for 2024.
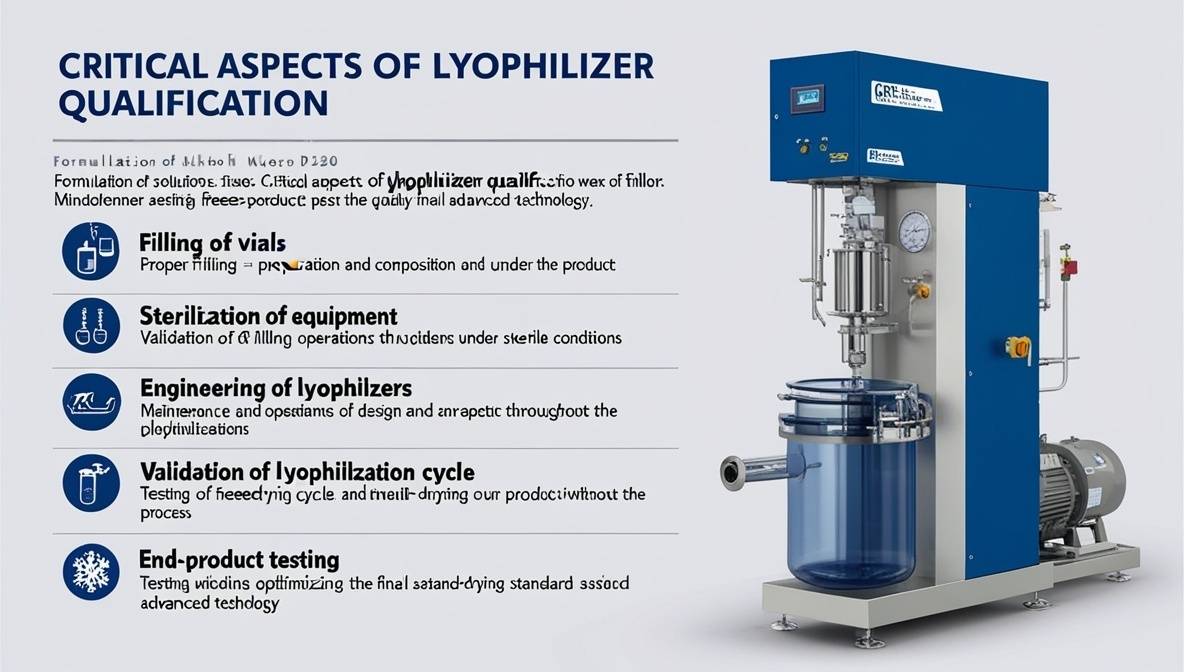
Critical Aspects of Lyophilizer Qualification
- Formulation of Solutions: Proper preparation and composition of the product.
- Filling of Vials: Validation of filling operations under sterile conditions.
- Sterilization of Equipment: Ensuring aseptic handling throughout the process.
- Engineering of Lyophilizers: Maintenance and design considerations.
- Validation of Lyophilization Cycle: Testing and optimizing the freeze-drying cycle.
- End-Product Testing: Ensuring the final product meets quality standards.
Industry Challenges
- Complex technology and equipment involved in manufacturing lyophilized pharmaceuticals.
- Deficiencies identified in industry inspections include sterility and potency issues.
- Guidance is crucial for investigators to manage and mitigate risks effectively.
- Challenges and Solutions: The process requires precise control to avoid issues such as product collapse or vacuum pump failure. Our troubleshooting guide addresses common problems faced during lyophilization.
- cGMP and Regulatory Compliance: Adhering to cGMP guidelines ensures product safety and quality. For detailed insights, refer to our cGMP guide and regulatory compliance practices.
- Validation and Qualification: Validation is critical to guarantee consistent results. Discover best practices for validation and the importance of operational qualification.Cryoprotectants in Lyophilization: Using cryoprotectants can preserve delicate structures. Learn more in this comprehensive guide.
Product Type/Formulation and Filling
- Purpose of Lyophilization:
- Used for products unstable in solution, e.g., semi-synthetic penicillins, cephalosporins, and salts of erythromycin, doxycycline, and chloramphenicol.
- Antibiotic Formulations:
- Typically exhibit low bioburden due to inherent antibacterial properties.
- Non-Antibacterial Products:
- Examples include hydrocortisone sodium succinate and biotechnology-derived products, requiring stringent microbiological control.
- Bioburden Control:
- Bioburden testing should be performed before sterilization to minimize endotoxin risks.
- Bulk solutions should be compounded in controlled environments and sealed tanks to prevent microbial growth.
- Testing Procedures:
- Prefiltration testing for gram-negative organisms, like Pseudomonas sp., helps assess contamination risks.
- Larger sample sizes (e.g., 100 mL) are more effective in detecting bioburden than smaller ones (e.g., 0.1 mL).
Filling
- Challenges in Filling for Lyophilization:
- Stoppering is done in the lyophilizer, leaving vials exposed to contamination until sealed.
- Validation Practices:
- Media fills and environmental monitoring during dynamic filling conditions are essential.
- Personnel evaluation includes monitoring gloves and gowns for microbial contamination.
- Manual Stoppering Concerns:
- Hand-stoppering, even with sterile forceps, is discouraged due to high contamination risks, except for clinical or small batch operations.
- Environmental Controls:
- Use of laminar flow barriers during filling, transport, and loading minimizes contamination risks.
- Proper design of airflows and laminar flow hoods is critical; improper setup can cause rebound contamination. and to maintain the air defferential air pressure of filling to controlled area
- Media Fill Studies:
- Used to validate aseptic capabilities, covering filling, transport, and loading into the lyophilizer.
- Expanded media fills segment the process to identify contamination points (e.g., filling, nitrogen flush, loading).
- Batch Size Considerations:
- Media fills should match the maximum batch size if less than 3000 units.
- Loading Process:
- Metal trays are used to transport vials to the lyophilizer, but trays should be removed before lyophilization to avoid moisture issues caused by warping.
- Facility Design:
- Filling lines should be close to the lyophilizer, with primary barriers extending between them.
- Vertical laminar flow hoods may be required but must be carefully designed to avoid air current-induced contamination.
Lyophilization Cycle and Controls
Lyophilization Cycle
- Initial Steps:
- Sterilize the lyophilizer and conduct aseptic loading.
- Freeze the solution, either by pre-cooling shelves or loading product before cooling.
- Freezing Challenges: or troublshooting of freeze drying process
- Ice formation on pre-cooled shelves may cause partial or complete stoppering of vials.
- Improper freezing rates (slow freezing) affect ice crystal size and product quality.
- Primary Drying:
- Maintain product temperature 4-5°C below the eutectic point.
- Ensure proper instrumentation for monitoring shelf temperature, product temperature, condenser temperature, and chamber pressure.
- Secondary Drying:
- Parameters such as shelf temperature, drying time, and pressure must be pre-defined and controlled.
- Process Monitoring:
- Continuous adjustments during the cycle indicate non-validated processes.
- Computer-controlled systems require operator involvement for loading, probe insertion, and parameter setup.
Cycle Validation
- Importance:
- Validation ensures reliability and identifies acceptable limits for key parameters.
- Knowing the eutectic point is crucial for evaluating abnormal cycles.
- Scale-Up Challenges:
- Variables such as freezing rate and temperature ramping affect product quality.
- Cycle parameters should be tailored for different strengths, vial sizes, and batch sizes.
Lyophilizer Sterilization and Leak Detection
- Sterilization Issues:
- Older lyophilizers often cannot tolerate steam sterilization, relying on chemical agents.
- Leak Detection:
- Leaks can originate from vacuum systems, thermal fluid, refrigerants, or vacuum pumps.
- Leak tests should be performed after sterilization and validated to ensure system integrity.
- Preventing Oil Vapor Migration:
- Employ oil traps or tortuous paths between vacuum pumps and chambers.
Equipment Malfunctions
- Common Issues:
- Malfunctions include power failures, equipment breakdown, or leaks.
- Proper corrective actions and documentation are necessary for quality assurance.
- Impact on Product:
- Contamination or sterility breaches can lead to batch rejection.
- Preventive Maintenance Logs and Discrepancy Reports should be reviewed during inspections.
Recommendations
- Use validated processes with well-defined parameters.
- Maintain and calibrate lyophilizer instrumentation regularly.
- Monitor product temperature, pressure, and other critical variables for quality assurance.
- Inspect manufacturing records and validate cleaning procedures for contamination prevention.
Finished Product Testing for Lyophilized Products
Dose Uniformity
- USP Standards:
- USP specifies two dose uniformity tests: content uniformity and weight variation.
- Applicability:
- Weight variation is applicable for solids prepared from true solutions and freeze-dried in final containers.
- Weight variation may also apply when excipients or additives are present, provided there’s a correlation between sample weight and potency.
- Correlation of Results:
- Potency results from assays should be correlated with the sample weight for accurate dose uniformity analysis.
- Assaying a vial without knowing its weight provides potency information but lacks dose uniformity data.
Stability Testing
- Moisture Concerns:
- Moisture content in vials is critical for product stability.
- Moisture specifications should be based on the worst-case data for product release and stability.
- Testing Requirements:
- Stability tests should involve vials with known sample weights.
- Higher fill weights may result in higher potency results, which may not represent the batch’s potency accurately.
- Expiration Date:
- Expiration dates and stability data should reflect batches with the highest moisture content.
- Potency specifications include:
- Higher limits at the time of product release.
- Compendial standards applicable throughout the expiration period.
- Aged Sample Assays:
- Stability tests should include aged sample assays and reconstitution potency tests for the maximum time specified on the label.
- Include tests for both the most and least concentrated reconstituted solutions.
- Highly concentrated solutions degrade faster than less concentrated ones.
Sterility Testing
- Reconstitution Solution:
- Use Sterile Water for Injection (WFI) instead of Bacteriostatic Water for Injection for sterility tests.
- Bacteriostatic Water can mask contamination by killing vegetative cells, leading to inaccurate results.
- Hospital Practices:
- Many hospitals prefer WFI due to potential toxicities associated with Bacteriostatic Water.
- Review of Contamination:
- Initial sterility test results showing contamination must be reviewed and contaminants identified.
Finished Product Inspection
Inspection Standards
- USP Recommendation:
- Perform 100% inspection of parenteral products, including sterile lyophilized powders.
- Critical Aspects:
- Inspect for the correct volume of the cake.
- Evaluate the cake’s appearance for any abnormalities.
Cake Appearance
- Meltback Issue:
- Meltback is a form of cake collapse caused by incomplete sublimation, where the solid changes to a liquid instead of vapor.
- This results in:
- Changes in the drug’s physical form.
- Presence of pockets of moisture.
- Consequences include reduced stability and increased degradation of the product.
Solubility Problems
Reconstitution Challenges: Poor solubility may lead to increased time for reconstitution during use. Partial dissolution can result in potency loss if the drug is not fully dissolved.
In-line Filters: During administration, in-line filters can cause additional drug loss if solubility issues are present.
Stability Considerations
- Meltback Impact: Partial or complete meltback impacts stability, especially in sensitive drugs like cephalosporins.
- Crystalline vs. Amorphous Form: Crystalline form is more stable than the amorphous form. The amorphous form often exists in the meltback portion of the cake, reducing product quality.
Atmosphere and the Earth
- The atmosphere is a gaseous envelope surrounding Earth, composed of:
- 78% nitrogen.
- 21% oxygen.
- Trace amounts of hydrogen, carbon dioxide, noble gases, water vapor, pollutants, and dust.
- It exerts pressure due to gravity, crucial for life and environmental processes.
Key Terms and Processes
Atmospheric Pressure
- Defined as the force exerted by the atmosphere at Earth’s surface.
- Standard reference: 760 torr (or millimeters of mercury).
Backstreaming: A low-pressure process where hydrocarbon vapors from the vacuum system enter the product chamber, risking contamination.
Blank-off Pressure: Represents the ultimate vacuum pressure a pump or system can achieve.
Blower (Mechanical Booster Pump)
- Positioned between the mechanical pump and chamber.
- Reduces chamber pressure to below 20 microns using high-speed rotating lobes.
Breaking Vacuum: Introducing air or specific gases into an evacuated chamber to restore pressure gradually.
Equipment and Components
- Circulation Pump:Used to convey heat transfer fluid for temperature regulation.
- Condenser (Cold Trap): Collects and freezes moisture during lyophilization. Protects the vacuum pump from water vapor contamination.
- Condenser/Receiver: Converts refrigerant gas into liquid and stores it for reuse in the refrigeration system.
- Conax Connection:A device ensuring a vacuum-tight seal when passing thermocouple wires through vessels.
Processes and Characteristics
- Contamination: Occurs when water vapor enters vacuum pump oil, affecting its efficiency.
- Defrosting: Removal of ice from a condenser using heat or mechanical means.
- Degree of Crystallization: Measures the energy released during freezing compared to water.
- Drying: Removes moisture or liquids through evaporation.
- Eutectic Temperature: The specific temperature where multiple phases coexist without change.
Freeze-Drying and Vacuum Processes
- Freezing: A controlled cooling process ensuring a completely frozen product.
- Gas Bleed: Introduces gas into the chamber to regulate pressure and improve heat transfer.
- Heat Exchanger: Transfers heat between the circulation and refrigeration systems.
- Hot Gas Defrost: A refrigeration technique to defrost condenser plates post-cycle.
- Inert Gas: Chemically inactive gases like nitrogen or helium used for specific applications.
Noteworthy Points
- Free Water: Moisture on product surfaces that must be removed for stability.
- Heat Transfer Fluid: A safe liquid used in systems to transfer heat efficiently.
- Interstage: A crossover in two-stage compressors, connecting low and high pressure zones.
Interstage Pressure Regulating Valve
This valve plays a critical role in controlling the interstage pressure of refrigeration or vacuum systems, ensuring it remains between 80 to 90 PSI. It opens to suction when the pressure exceeds this range, maintaining system safety and efficiency.
Lexsol
A high-grade kerosene commonly used as a heat transfer fluid in industrial systems due to its excellent thermal conductivity and stability.
Liquid Sub-Cooler Heat Exchanger
This device sub-cools the liquid refrigerant leaving the condenser, reducing its temperature to between +15°F (-10°C) and -15°F (-25°C). Sub-cooling enhances refrigerating efficiency and minimizes vapor formation during the process.
Lyophilization
Lyophilization is a two-stage process where a product is first frozen and then subjected to sublimation and desorption in a vacuum. This technique removes water while preserving the product’s structure and limiting biological and chemical reactions for long-term storage.
Main Vacuum Valve and matrix
Located between the chamber and the external condenser, this valve isolates the two components post-process. It safeguards the finished product from potential contamination. Matrix In lyophilization, a matrix refers to a network of ice crystals and solids distributed uniformly within the frozen product, ensuring stability during sublimation.
Mechanical Booster Pump
Also known as a blower or roots pump, this system has a high displacement and low compression ratio. When combined with an oil-sealed rotary pump, it provides an efficient alternative for achieving high vacuum levels economically.
Melting Temperature (Melt-back)
This is the temperature at which mobile water becomes evident in a frozen product, signifying the start of melting.
Non-Condensables and Nucleation
These are unwanted gases, such as nitrogen, hydrogen, and hydrocarbons, that enter the system through leaks. Their presence increases condensing pressure, reducing system efficiency. Nucleation The initial formation of ice crystals triggered by foreign surfaces or water clusters, crucial for achieving a uniform freezing process.
Oil-Mist Filter
A filter attached to the exhaust of an oil-sealed rotary pump to trap suspended oil droplets, preventing environmental contamination.
Sublimation or primary drying stage
The phase change where a solid (ice) directly transitions into vapor without becoming liquid. It forms the primary drying stage of lyophilization, where most of the moisture is removed.
Vacuum Pump
The vacuum pump is a mechanical device that reduces chamber pressure to sub-atmospheric levels, enabling sublimation. It includes various types, such as rotary vane, rotary piston, and mechanical booster pumps.
Two-Stage Compressor
A specialized compressor designed to achieve low temperatures and pressures. It combines two compressors—one for low-stage operations and another for high-stage—connected via interstage piping.
Vapor Valve or isolation volve or solenoid volve
Also called the main vapor valve, this component isolates the chamber from the external condenser by closing off the vacuum path, critical for maintaining chamber integrity.
Virtual Leak
In vacuum systems, this refers to trapped gases escaping into the chamber from internal surfaces, potentially impacting system performance.
Vial
A small glass bottle with a flat bottom, short neck, and flat flange designed for secure stoppering, commonly used in pharmaceutical applications.
Summary Lyophilization qualification guidelines
Lyophilization, or freeze-drying, is a crucial process for producing stable, dry pharmaceutical products. It involves freezing, primary drying, and secondary drying stages to remove water without compromising product integrity. This guide outlines the advantages and challenges of lyophilization, highlights key steps in the process, and provides detailed guidance on lyophilizer qualification, including formulation, sterilization, and validation practices. Special emphasis is placed on maintaining aseptic conditions, ensuring equipment integrity, and addressing challenges such as bioburden control and environmental contamination.
Conclusion of Lyophilization qualification guidelines
Lyophilization is a vital technique in pharmaceutical manufacturing, offering stability and ease of handling for sensitive products. However, the process requires meticulous qualification and validation to ensure product quality and sterility. By adhering to proper guidelines for equipment maintenance, process validation, and environmental control, manufacturers can overcome challenges and achieve consistent results. This guide serves as a comprehensive resource for understanding the intricacies of lyophilization and the critical factors for successful implementation.
FAQs
What is lyophilization, and why is it used?
Lyophilization, or freeze-drying, removes water from products to enhance stability and ease of handling. It is commonly used for pharmaceuticals and biological products sensitive to heat and moisture.
What are the key stages of the lyophilization process?
The process involves three stages:
-
- Freezing: Crystallizes the product.
- Primary Drying (Sublimation): Removes frozen water under vacuum.
- Secondary Drying (Desorption): Eliminates bound water molecules.
What are the main challenges in lyophilization?
Challenges include high equipment costs, the need for strict aseptic conditions, and process complexity, such as preventing contamination during filling and maintaining equipment sterility.
How is lyophilizer qualification performed?
Qualification involves validating formulation preparation, sterilization processes, filling operations, lyophilization cycles, and end-product testing to ensure compliance with quality standards.
What are the common equipment issues during lyophilization?
Equipment issues include leaks, power failures, and sterilization difficulties. Preventive maintenance and thorough documentation are essential to mitigate these risks and ensure system integrity.