Understanding Lyophilization Process
lyophilization process, often referred to as freeze-drying, is a crucial process in various industries.
- History of Lyophilization: The technique traces its roots back to ancient times, but it gained prominence during World War II for preserving blood plasma.
- Importance of Lyophilization: It is indispensable in preserving sensitive biological materials and heat-sensitive pharmaceuticals.
Process of Lyophilization
Freezing Phase
Freezing phase In this initial phase, the product is frozen to a temperature below its triple point.
- Lyophilization, also known as freeze-drying, is an important process in various industries.
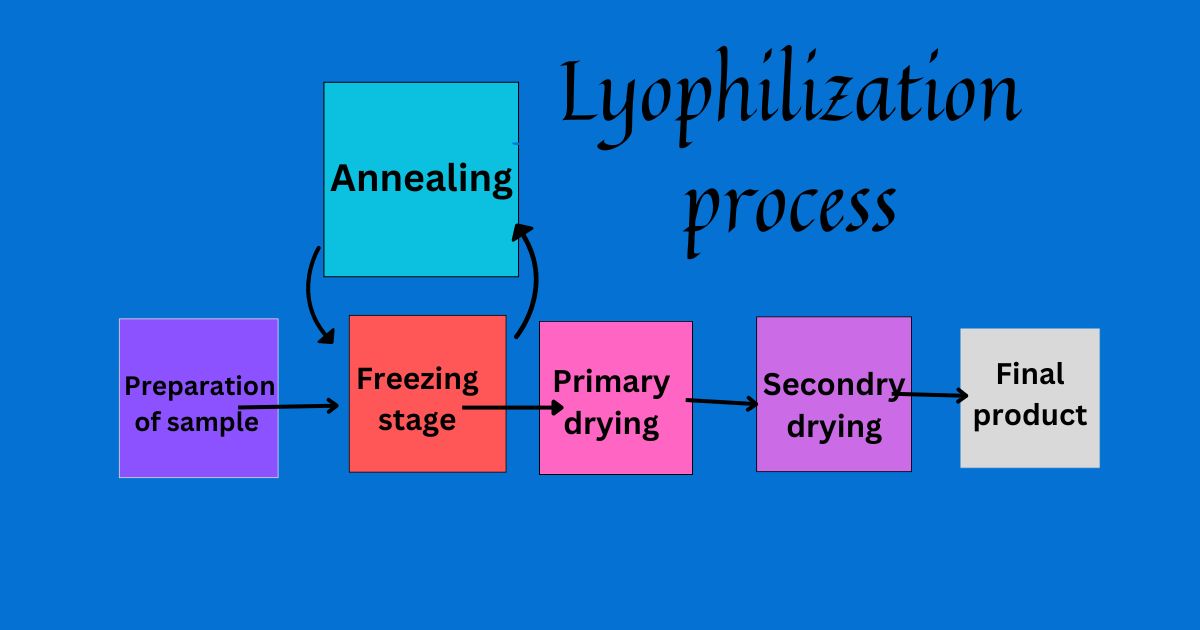
- Sample preparation is essential before starting the lyophilization process.
- Freezing initiates the process of ice crystal formation and the stepwise separation of solutes.
- Primary drying removes water vapor driven by a temperature gradient.
- It is important to maintain the primary drying temperature below the critical temperature to prevent adverse effects.
- Determining the critical temperature is important to optimize the lyophilization cycle.
- Annealing, an optional step, can increase product stability, especially for crystalline materials.
Secondary drying removes water molecules bound to the product. - Monitoring critical parameters and ensuring regulatory compliance is part of quality control.
Lyophilization has applications in pharmaceuticals, the food industry, and biotechnology. - There are equipment limitations, cost factors, and regulatory compliance challenges.
- Advances in technology and research may lead to improvements in lyophilization processes.
- The freezing phase is the initial step in the lyophilization process.
- Methods such as shelf freezing, liquid nitrogen immersion, or controlled-rate freezing can be used.
- The goal is to solidify the water content within the product while minimizing large ice crystal formation.
- Adequate temperature control and uniform cooling are essential.
- The freezing phase prepares the product for subsequent drying phases.
Primary Drying Phase
Primary Drying Phase: During primary drying, ice sublimates directly from the solid to the vapor phase.
- Primary Drying:
- Pressure is reduced below the triple point of water.
- Heat is supplied to provide the latent heat of sublimation.
- It typically takes around 9 to 10 hours.
- Removes about 95% of water in crystalline eutectic systems.
- Situation during primary drying:
- Vaporized water leaves the solid and is removed to a condenser.
- Maintains low vapor pressure in the chamber.
- Forms a layer of dried material as water is removed.
- The sublimation front moves down through the depth of the drying material.
Secondary Drying Phase
Secondary Drying Phase: This phase removes any remaining unfrozen water molecules, ensuring the product’s stability.
- Secondary drying (adsorption)
- Removes bound water molecules.
- Temperature is raised higher than in primary drying.
- Bonds between material and bound water molecules are broken.
- Temperatures are typically between 10°C and 50°C.
- Requires a long duration.
- The completion of secondary drying leaves the sample with only about 1%–2% water.
- Applications of Emulsified Materials:
- Used for spray- and freeze-drying microencapsulation.
- Incorporated into the textile fiber matrix during the extrusion process.
- Act as templates for the coacervation process.
Benefits of the Lyophilization Process
Benefits of the Lyophilization Process: it retains the product’s integrity, enhances stability, and allows for long-term storage.
Applications of Lyophilization
Pharmaceutical Industry
Lyophilization is extensively used for preserving vaccines, antibiotics, and biologics. Here are the applications of lyophilization in the pharmaceutical industry:
- Preservation of labile drugs and biologics by stabilizing them in a dry state.
- Extended shelf life of pharmaceutical products, reducing waste, and improving inventory management.
- Facilitation of long-term storage and transportation of vaccines and other temperature-sensitive medications.
- Production of lyophilized injectable formulations, ensuring rapid reconstitution and administration.
- Preservation of diagnostic reagents and enzymes, maintaining their activity and efficacy.
- Preparation of stable oral solid dosage forms, such as lyophilized tablets and capsules.
- Lyophilization of biopharmaceuticals, including proteins, antibodies, and peptides, for enhanced stability and bioavailability.
- Encapsulation of probiotics and live biotherapeutic products (LBPs) for improved stability and efficacy.
- Production of lyophilized dosage forms for pediatric and geriatric patients, offering ease of administration and accurate dosing.
- Development of lyophilized formulations for personalized medicine and targeted drug delivery applications.
Food Industry
Here are the applications of lyophilization in the food industry:
Biotechnology
Lyophilization is vital for preserving enzymes, probiotics, and cell cultures. Here are the applications of lyophilization in biotechnology:
- Preservation of enzymes, antibodies, and other biomolecules for research, diagnostics, and therapeutic applications ensures long-term stability and activity.
- Lyophilization of cell cultures and microbial strains for bioprocessing, storage, and distribution, maintaining viability and genetic integrity.
- Production of lyophilized reagents and media formulations for bioproduction processes, facilitating scalable and reproducible manufacturing of biopharmaceuticals.
- Encapsulation of biologically active compounds and nanoparticles for targeted drug delivery systems enhances stability and bioavailability.
- Preparation of stable vaccines and diagnostic kits through lyophilization, enabling long-term storage, distribution, and global health initiatives.
Challenges and Solutions
- Moisture Control: Controlling moisture content is critical to preventing degradation.
- Product Stability: Ensuring stability throughout the process is essential for maintaining efficacy.
- Equipment Constraints: Selecting appropriate equipment and optimizing parameters mitigate challenges.
Alarm observed during freeze drying process
- Failure of all vacuum pump
- Failure of all vacuum pump during evacuation
- failure of the active MKS AND PIRANI chamber pressure sensor
- Failure of booth vacuum pressure sensor in the chamber
- loss of power during freeze-drying process
- failure of the temperature sensor in the shelf intake
- Failure of refrigerating compressor during condenser cooling
- failure of silicon heater
- Failure of the all-active silicon oil pump
Future Trends in Lyophilization
- Technological Advancements: Advanced equipment and process automation are revolutionizing lyophilization.
- Automation: Automated systems streamline operations, reducing manual intervention.
- Sustainability: Efforts to minimize energy consumption and waste are shaping the future of lyophilization.
Safety Considerations
- Contamination Risks: Maintaining sterile conditions is imperative to prevent contamination.
- Regulatory Compliance: Adhering to regulatory standards ensures product safety and efficacy.
- Best Practices for Safe Lyophilization: Implementing stringent protocols and quality control measures is paramount.
Cost-effectiveness of lyophilization
- Economic Benefits: Despite initial investment, the long-term benefits justify the cost.
- Return on Investment: Lyophilization offers substantial returns by extending product shelf life and minimizing losses.
Case Studies
- Successful Implementation Examples: Case studies highlight successful lyophilization applications across industries.
- Lessons Learned: Analyzing past experiences provides insights for optimizing future processes.
Conclusion of the Lyophilization Process
Frequently asked questions about the lyophilization process
What are the steps in the lyophilization process?
The lyophilization process generally involves three main steps: pre-freezing, primary drying, and secondary drying.
What is the principle of lyophilizing?
The principle of the lyophilizer, also known as a freeze dryer, is based on the sublimation of frozen water from a product under vacuum conditions, preserving the structure and characteristics of the product.
What are the steps to freeze-drying?
Freeze-drying involves pre-freezing the product, followed by primary drying (sublimation of frozen water) and secondary drying (removal of residual moisture).