Understanding Glass Vials in Pharmaceutical Freeze-Drying
Glass vials stand as the primary packaging material in pharmaceutical freeze-drying processes. Glass vials are molded vials, and tubular vials are generally used for pharmaceutical freeze dryer This article delves into the manufacturing processes, types, and thermal parameters of these vials.
Introduction to Glass Vials in Pharmaceutical Freeze-Drying
Overview of Glass Vials
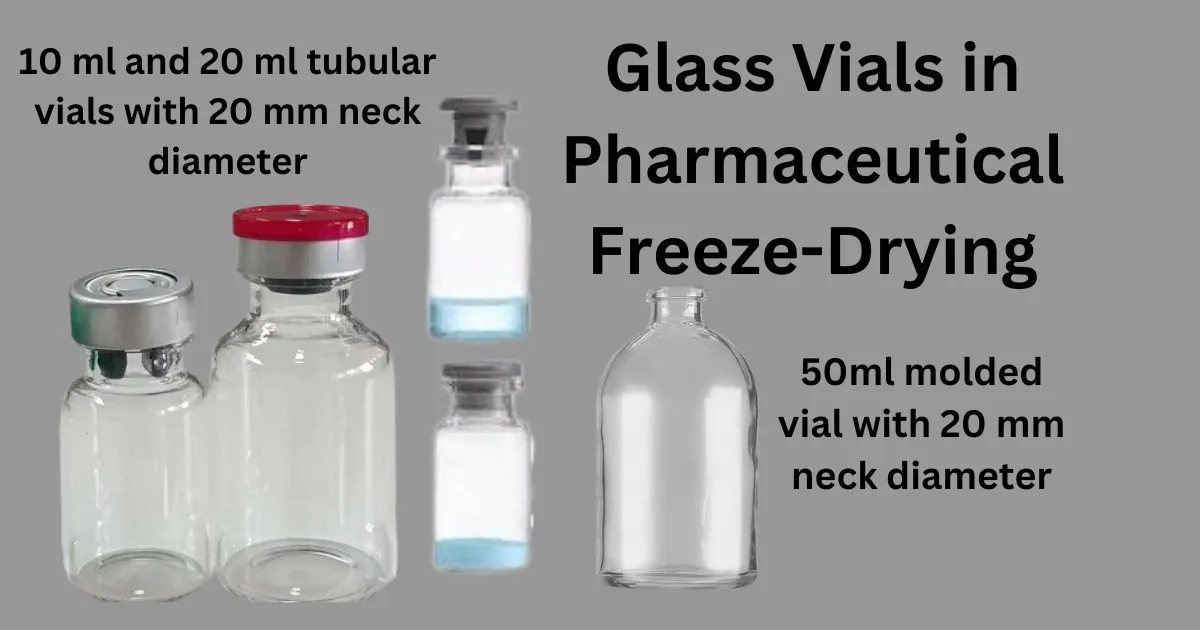
Glass vials hold significant importance in pharmaceutical packaging, particularly in freeze-drying applications. Among the varieties available on the market, tubing vials and molded vials are predominant.
Significance of Vial Type
The fill volume of the product influences the decision between tubing vials, tubular vials, and molded vials. Small-volume parenterals typically use tubing vials, while products with larger fill volumes are suited for molded vials.as like 50-ml molded vials, 30-ml molded vials, and 20-ml molded vials
Manufacturing Process of Glass Vials
Tubing Vials or tubular Vials
The manufacturing of tubing vials involves a two-step process, utilizing glass tubes as an intermediary outcome.
A tubular vial, also known as a tubing vial, is a type of glass vial used in pharmaceutical packaging. It is formed from a cylindrical glass tube, typically with uniform dimensions throughout its length. Tubular vials are commonly used for small-volume parenterals and are particularly suited for freeze-drying processes.
similar to a 10 ml tubular vial with a neck diameter of 13 mm and 2 ml tubular vial with 13 mm neck diameter, 10 ml tubular vials with 20 mm neck diameter are generally used for freeze drying process for small volumes of product
Molded Vials
Molded vials are also produced through a two-step process. Initially, molten glass is molded into an initial parison, which is then shaped into the final vial form by inflating it with compressed air.
For large filled volumes of freeze-dried product, 50-ml molded vials or 30-ml molded vials with a neck diameter of 20 mm are typically utilized.
Press Blow vs. Blow Blow
Two common processes in molded vial manufacturing are press blow (PB) and blow blow (BB). PB results in a more uniform glass weight distribution and wall thickness. Recent advancements have enabled the production of smaller PB-molded vials, even down to 15-mL injection vials.
Here is a summary of the key differences between press blow (PB) and blow blow (BB) in molded vial manufacturing
Here are “Thermal Parameters and Freeze-Drying Process.”
- Thermal parameters such as shelf temperature, chamber pressure, and product temperature play crucial roles in the freeze-drying process.
- The shelf temperature determines the heat transfer to the product, affecting the drying rate and product quality.
- Chamber pressure controls the sublimation of ice within the product, influencing drying time and efficiency.
- Product temperature monitoring ensures that the product remains within specified temperature ranges to prevent degradation or collapse during drying.
- Optimizing thermal parameters is essential to achieving efficient and uniform drying, resulting in high-quality freeze-dried products with extended shelf life.
Importance of Thermal Parameters
Efficient heat transfer is crucial throughout the freeze-drying process to ensure optimal product quality.
Heat Transfer Coefficient
The heat transfer coefficient describes the rate of energy transfer between the freeze-dryer and the container system, playing a vital role in the Quality by Design (QbD) approach.
The following are key details about the heat transfer coefficient and how the Quality by Design (QbD) methodology uses it:
- The heat transfer coefficient quantifies the rate of energy transfer between the freeze-dryer and the container system during the freeze-drying process.
- Understanding and optimizing the heat transfer coefficient is crucial for achieving consistent and reproducible drying rates and ensuring uniform product quality.
- Incorporating the heat transfer coefficient into the Quality by Design (QbD) approach allows for a systematic understanding of its impact on product characteristics and process performance.
- By controlling and enhancing the heat transfer coefficient, manufacturers can improve process efficiency, reduce cycle times, and minimize energy consumption in freeze-drying operations.
- Using QbD principles to figure out and improve the heat transfer coefficient makes it easier to create freeze-drying methods that are reliable, scalable, and meet safety and effectiveness standards.
Vial Heat Transfer Coefficient (Kv)
Kv values are essential for developing or transferring freeze-drying cycles. They aid in determining the design space and can reduce the number of experiments required for successful cycle transfer.
The following are key points about Kv values and how they relate to creating or transferring freeze-drying cycles:
- Kv values, also known as the heat transfer coefficient or vial heat transfer coefficient,. Quantify the heat transfer efficiency between the freeze-dryer shelf and the vial or container system.
- These values are essential for accurately predicting and controlling the temperature distribution within the product during the freeze-drying process.
- Kv values play a critical role in developing freeze-drying cycles by informing decisions regarding shelf temperature, chamber pressure, and drying time.
- When transferring freeze-drying cycles between different freeze-dryers or facilities,. Kv values provide valuable insight into the heat transfer characteristics of the system, ensuring process consistency and product quality.
- Optimizing Kv values allows manufacturers to achieve uniform drying rates. Minimize drying time variability and enhance product quality and stability during freeze-drying operations.
Conclusion
In conclusion, glass vials play a crucial role in the pharmaceutical freeze-drying process, offering solutions tailored to different product volumes. Understanding the manufacturing processes, such as tubing and molded vials, alongside key thermal parameters is essential for optimizing freeze-drying cycles. By delving into the interplay between vial types, manufacturing techniques, and thermal considerations. Pharmaceutical professionals can ensure efficient and effective freeze-drying operations, ultimately leading to high-quality products with extended shelf life and enhanced stability.
FAQs
What are the main types of glass vials used in pharmaceutical freeze-drying?
Tubing vials and molded vials are the primary types utilized, each catering to different fill volumes of pharmaceutical products.
What is the significance of the vial type in freeze-drying?
The choice between tubing vials and molded vials depends on the fill volume of the product. With small-volume parenterals suited for tubing vials and larger fill volumes for molded vials.
What are the advantages of the press blow (PB) process in molded vial manufacturing?
PB results in a more uniform glass weight distribution and wall thickness, contributing to better product quality.
How do thermal parameters impact the freeze-drying process?
Efficient heat transfer is essential for successful freeze-drying, ensuring optimal product quality and process efficiency.
Why are Kv values important in freeze-drying cycle development?
Kv values help with Quality by Design (QbD). Method by making it easier to create or move freeze-drying cycles and lowering the number of tests that need to be done to make sure the cycle works.