🔬Eutectic Temperature in Lyophilization: Understanding the Critical Point in Freeze-Drying
Freeze-drying, scientifically known as lyophilization, is a crucial process in various industries, including pharmaceuticals, food preservation, and biotechnology. At the heart of this process lies the concept of eutectic temperature, a critical factor that significantly influences the success of the lyophilization process.
👉 Related: What is Lyophilization Technology?
🧊What is lyophilization?
Lyophilization is a dehydration process that involves removing moisture from a product while preserving its structure and properties. It consists of three main steps: freezing, primary drying (sublimation), and secondary drying (desorption). This technique is favored for its ability to extend the shelf life of products, maintain stability, and facilitate easy storage and transport. For a step-by-step overview, see Freeze-Drying Process Steps Explained Clearly
🔄Steps involved in Lyophilization
- Loading stage
- Freezing
- Primary drying and secondary drying
- Venting temperature
- Venting before stoppering
- Stoppering stage
- Unloading temperature
- Unloading stage
🌡 Understanding Eutectic Temperature
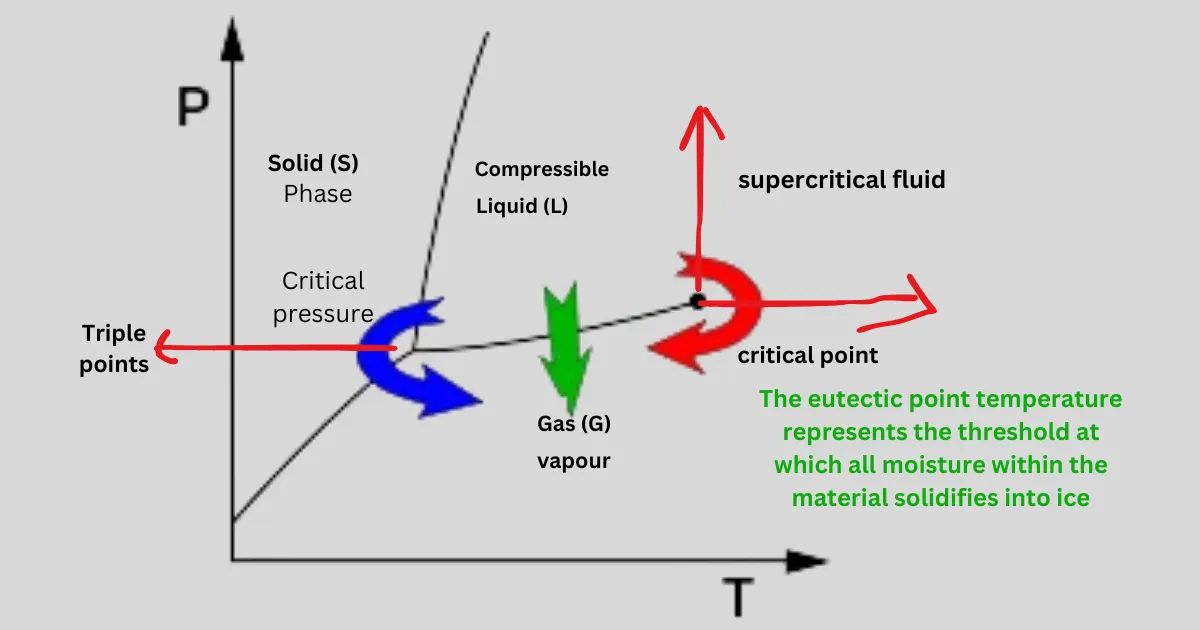
The eutectic temperature refers to the lowest temperature at which the eutectic mixture in a system becomes completely liquid. In the context of lyophilization, it is the temperature at which the eutectic solution present in the product undergoes phase transition, posing a risk of product collapse or degradation if not managed properly.
To learn more about monitoring temperature thresholds, explore Temperature Control in Freeze-Drying Processes
The eutectic point temperature represents the threshold at which all moisture within the material solidifies into ice. In manufacturing research, to ensure thorough freezing of the material, a temperature typically 5-10°C below the eutectic point is commonly chosen for the freezing process.
📉 Identifying the Eutectic Point and Co-Melting Point
- Determination of eutectic point and co-melting point
- Importance of eutectic point and co-melting point in freeze-drying process
- DSC method used for determining eutectic point and co-melting point
- Process of determining eutectic point and co-melting point using DSC method
- significance of pre-freezing temperature in freeze-drying process
- Effects of setting pre-freezing temperature too high or too low
- Determination of pre-freezing temperature for Tremella fuciformis soup material
- Definition and significance of co-melting point temperature
- importance of not exceeding co-melting point temperature during sublimation drying stage
- Strategies for ensuring the quality of dry products during the sublimation drying stage.
For practical insights, refer to Pre-Study Qualification of Thermocouple
⚠️ Collapse Temperature in Lyophilization
Collapse temperature (Tc) is a critical parameter in the lyophilization (freeze-drying) process, particularly for amorphous or partially amorphous products. It refers to the temperature at which a product loses its structural integrity during primary drying due to the softening or “collapse” of its matrix. When the product temperature exceeds Tc, it can lead to poor cake appearance, shrinkage, and loss of porosity, which negatively affects reconstitution time and product stability.
Learn more here: Impact of Temperature Overshoots During Lyophilization
Also explore: Dry Layer Resistance During Primary Drying

Additionally, proper equipment care, such as freeze dryer operational qualification and performance testing, is essential to maintaining ideal conditions throughout the lyophilization process.
✅ Importance of Eutectic Temperature in Lyophilization
The eutectic temperature plays a crucial role in determining the optimal conditions for freeze-drying various products. During the freezing stage of lyophilization, water molecules in the product form ice crystals, along with solutes such as sugars, proteins, or other components. When the temperature reaches the eutectic point, these solutes dissolve in the melted ice, creating a eutectic solution. Related article: Lyophilization Temperature Guidelines
🧪 Challenges Posed by Eutectic Temperature
The presence of eutectic solutions can pose challenges during the lyophilization process. If the temperature is not carefully controlled, the eutectic solution may undergo phase separation, leading to collapse or structural damage of the product. This can result in decreased product efficacy, altered physical characteristics, or even complete loss of the product. Explore real-world impacts here: Defects in Lyophilized Product – A Complete Easy Guide

🔧 Managing Eutectic Temperature in Lyophilization
To mitigate the risks associated with eutectic temperature, precise control of the lyophilization process is essential. This includes monitoring and adjusting the freezing parameters, such as cooling rate and holding time, to minimize the formation of eutectic solutions. Additionally, selecting appropriate excipients or formulation strategies can help stabilize the product and prevent eutectic phase separation. Refer to Lyophilization Cycle Development Guide for Success
Applications of Eutectic Temperature Knowledge
Understanding the eutectic temperature is vital for optimizing lyophilization processes across various industries.
- In pharmaceuticals, it ensures the stability and efficacy of drugs and vaccines.
- In the food industry, it preserves the quality and flavor of perishable products.
- In biotechnology, it facilitates the preservation of biological samples and reagents.
Discover broader uses: Applications of Freeze-Drying in Biopharmaceuticals
🧾 Conclusion
Eutectic temperature is a critical parameter in the lyophilization process, influencing the success and quality of the final product. By understanding its significance and implementing proper control strategies, manufacturers can ensure the integrity, stability, and efficacy of lyophilized products across diverse applications.
In essence, mastering the eutectic temperature in lyophilization is essential for unlocking the full potential of this versatile dehydration technique, paving the way for innovation and advancement in various industries.
Enhance your freeze-drying knowledge:
👉 Lyophilization Validation Best Practices 2025
👉 Freeze Dryer Operational Qualification Protocol
❓ FAQs – Eutectic Temperature in Freeze-Drying
Why is the eutectic point important?
The eutectic point is important because it influences the freezing behavior and the structure of the freeze-dried product, impacting its physical properties and stability.
What is the eutectic temperature when frozen?
The eutectic temperature of freezing is the lowest temperature at which the eutectic mixture forms during the freezing process. It represents the point at which the solution transitions from the liquid to the solid phase.
What is the temperature of the eutectic line?
The temperature of the eutectic line indicates the temperature range over which the eutectic mixture remains stable during the freezing process, ensuring uniform freezing and consistent product quality.
What happens at the eutectic point?
At the eutectic point, the components of the solution crystallize simultaneously, forming a eutectic mixture with a specific composition. This phenomenon can affect the physical properties and stability of the final lyophilized product.
What is eutectic temperature in freezing?
The eutectic temperature is the lowest temperature at which a mixture of substances can coexist in a liquid phase before solidifying into a crystalline structure. In freezing, it represents the temperature at which the solution solidifies completely.
What is the best temperature for lyophilization?
The best temperature for lyophilization (freeze-drying) varies depending on the specific material being dried. Generally, the primary drying phase is conducted below the eutectic or collapse temperature to ensure the product remains frozen while sublimation occurs. This typically ranges from -40°C to -20°C.
What is the collapse temperature in lyophilization?
The collapse temperature is the temperature at which a frozen product loses its structural integrity during the freeze-drying process. It is crucial to keep the product temperature below the collapse temperature to maintain the product’s structure and prevent collapse.
