In the realm of pharmaceuticals, biotechnology, and food preservation, the lyophilizer process plays a pivotal role in ensuring the stability and longevity of sensitive materials. Also known as freeze-drying, this technique involves removing moisture from products while preserving their structure and integrity.
Let’s delve deeper into the lyophilizer process, its applications, and the science behind its effectiveness.For an in-depth process overview, visit our guide on Understanding the Freeze-Drying Process.
Table of Contents
ToggleWhat is lyophilization?
Lyophilization is a method of drying substances that are sensitive to heat or pressure, such as proteins, vaccines, antibiotics, and certain food products. Unlike conventional drying methods, lyophilization involves freezing the material and then subjecting it to vacuum pressure, which allows the frozen water to sublimate directly from solid to vapor without passing through a liquid phase. This results in a product with minimal damage to its structure and composition. For an overview of the full steps, refer to our article on Freeze Drying Process Steps Explained Clearly.
Lyophilization, often referred to as freeze drying, is a method used to remove water from perishable materials, aiming to prolong shelf life or enhance transport convenience. The process involves freezing the material and subsequently reducing the pressure while applying heat to facilitate the sublimation of frozen water within the material.
Lyophilization vs Freeze Drying
Lyophilization, often referred to as freeze drying, is a method used to remove water from perishable materials, aiming to prolong shelf life or enhance transport convenience. The process involves freezing the material and subsequently reducing the pressure while applying heat to facilitate the sublimation of frozen water within the material.
To better understand the comparison, explore our post on Lyophilization vs Freeze Drying.
Summary
- Water sublimes from the solid to the vapor state under controlled temperature and pressure.
- This process preserves biological materials that are intact and active.
- Freeze-drying protectants include sugars, anti-oxidizing agents, and bulking materials.
- Sugars found in extremophiles act as natural protectants.
- Excipients, or carrier materials, form the majority of the mass in lyophilized products.
- Lyophilization finds applications in pharmaceuticals, food processing, and biotechnology.
What are the differences between a lyophilizer and a freeze dryer?
| Feature | Lyophilizer | Freeze-Dryer |
|---|---|---|
| Purpose | Used for lyophilization or freeze-drying processes. | Primarily designed for freeze-drying applications. |
| Operation | Operates under vacuum conditions. | Operates under vacuum conditions. |
| Temperature Control | Controls both freezing and drying temperatures. | Controls freezing and drying temperatures separately. |
| Pressure Control | Allows for precise pressure adjustment during the process. | Allows for precise pressure adjustment during the process. |
| Applications | Widely used in the pharmaceutical, biotechnology, and food industries. | It is generally used in laboratories and industrial settings for various applications. |
| Size | Available in various sizes, from benchtop to large-scale industrial units. | Available in different sizes, suitable for laboratory and industrial use. |
| Cost | Generally more expensive due to specialized design and functionality. | Cost-effective options are available for smaller-scale applications. |
This table outlines the key differences between a lyophilizer and a freeze dryer in terms of their operation, applications, and other features.
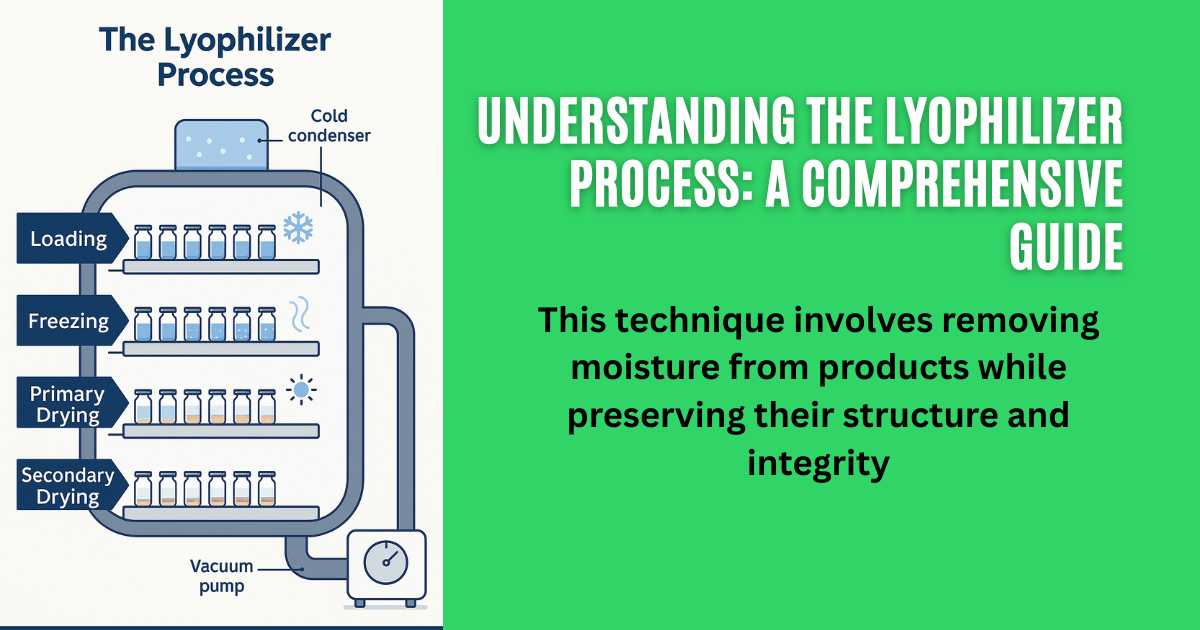
The Lyophilizer Process
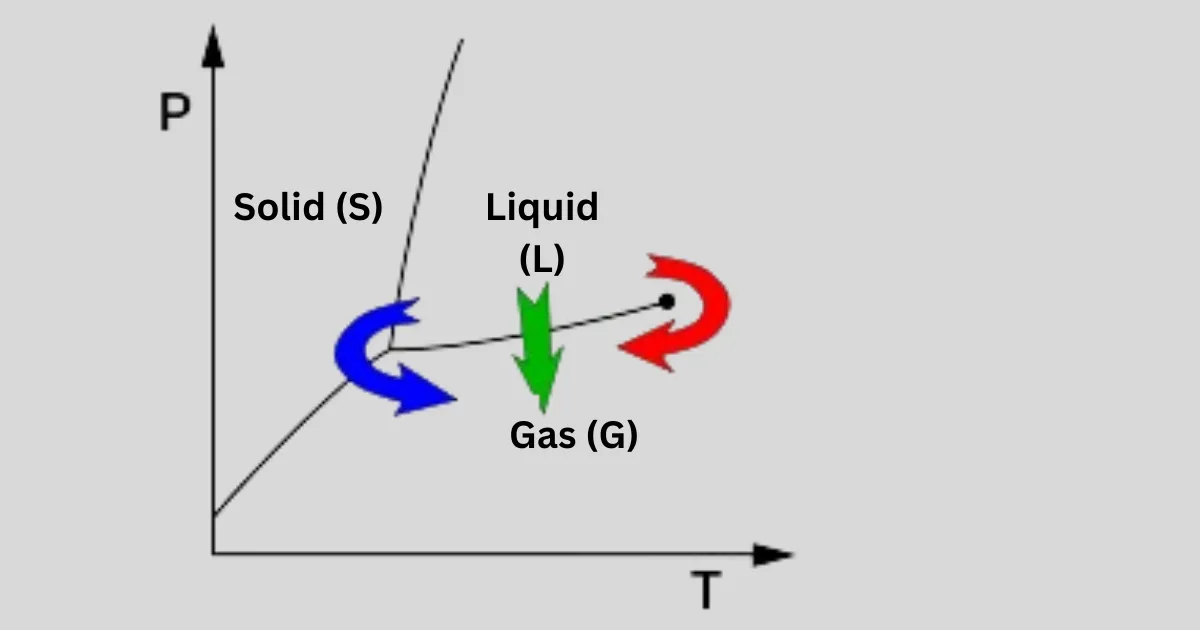
In a regular phase diagram, there’s a line marking the shift from gas to liquid, stretching from the triple point to the critical point. When we freeze-dry, as shown by the blue arrow, we guide the system close to the triple point, bypassing the direct transition from liquid to gas, as seen in normal drying processes, as indicated by the green arrow.
Loading stage
After starting the freeze dryer, the loading temperature achieved in the freezer, then the freeze dryer door is opened on aseptic area side, and the empty and filled vials are loaded in the freezer with the help of ALUS (Automatic loading and unloading system), and then the freezing cycle is started by SCADA Learn more in our detailed Freeze Dryer Shelf Package Installation Verification guide.
Loading stage: Once the freeze dryer is started and the loading temperature is reached, the door is opened in a sterile area. Empty and filled vials are then loaded into the freeze dryer before initiating the freezing cycle using SCADA.
Freezing
The first step in the lyophilizer process is to freeze the material. This is typically done by lowering the temperature of the product below its freezing point, causing the water molecules to form ice crystals. Explore Lyophilization Temperature Guidelines for correct parameter setup.
Summary
- Freezing the product can be achieved through various methods, such as using a freezer, a chilled bath (shell freezer), or placing it on a shelf in the freeze dryer.
- Cooling the material below its triple point ensures sublimation instead of melting, preserving its physical form.
- Large ice crystals, which can be generated through slow freezing or annealing, facilitate lyophilization.
- Large ice crystals can lead to cell wall breakage in biological materials, resulting in suboptimal freeze-drying outcomes.
- Rapid freezing is employed to prevent cell wall damage in biological materials during lyophilization.
- Annealing, a process that involves rapidly freezing the material followed by increasing its temperature, can help materials prone to precipitation by allowing crystals to grow.
Primary Drying
Primary Drying: Once the material is frozen, it is placed in a vacuum chamber, and the pressure is reduced. This low-pressure environment allows the frozen water to sublimate, meaning it transitions directly from ice to vapor without melting first. This phase is called primary drying and is crucial for removing the majority of the moisture from the product. We discuss this further in our article on Primary and Secondary Drying in Lyophilization.
Summary
- Primary drying, also known as sublimation, is the second phase of lyophilization.
- During primary drying, pressure is reduced, and heat is applied to facilitate water sublimation.
- The vacuum accelerates the sublimation process.
- A cold condenser helps water vapor adhere and solidify, protecting the vacuum pump.
- Approximately 95% of the water in the material is removed during primary drying.
- Primary drying can be slow, and excessive heat can potentially alter the material’s structure.
Secondary Drying
Secondary Drying: After primary drying, there is still residual moisture present in the product. To remove this moisture, the temperature is slightly increased, and a secondary drying phase begins. This ensures the product is thoroughly dry and stable. Understand more about moisture concerns in Residual Moisture Determination.
Summary
- The final phase of lyophilization is secondary drying, also known as adsorption.
- In secondary drying, ionically bound water molecules are removed by raising the temperature.
- Higher temperatures break the bonds between the material and water molecules.
- Freeze-dried materials maintain a porous structure.
- After lyophilization, the vacuum can be broken with an inert gas before sealing the material.
- Most materials can be dried to a residual moisture content of 1–5%.
Applications of Lyophilization
The lyophilizer process finds widespread applications across various industries.
Pharmaceutical Industry: Lyophilization is commonly used in the pharmaceutical industry to stabilize drugs, vaccines, and biologics. By removing moisture, lyophilization can increase the shelf life of these products and make them more stable for storage and transportation. Check our pharmaceutical-specific guide: Pharmaceutical Freeze Drying Q&A Guide.
Food Preservation: Many food products, such as fruits, vegetables, and dairy, are lyophilized to prolong their shelf life and maintain their nutritional value. Freeze-dried foods are lightweight, easy to store, and can be rehydrated quickly when needed. Learn more about Best Fruits for Freeze Drying.
Biotechnology: In biotechnology, lyophilization is used to preserve enzymes, antibodies, and other biomolecules. By removing water from these sensitive materials, lyophilization helps maintain their activity and integrity for research and diagnostic purposes. For biopharma applications, visit Applications of Freeze Drying in Biopharmaceuticals.
Conclusion
The lyophilizer process, or freeze-drying, is a sophisticated technique for preserving sensitive materials by removing moisture without causing damage. Its applications span across industries, from pharmaceuticals to food preservation and biotechnology. Understanding the science behind lyophilization is essential for ensuring the quality, stability, and efficacy of the final products. For more technical insight, refer to Lyophilized Process: A Comprehensive Guide.
(FAQs) about the Lyophilizer Process
What is a lyophilizer?
A lyophilizer, also known as a freeze dryer, is a piece of equipment used for the process of lyophilization, or freeze-drying. It removes moisture from sensitive materials while preserving their structure and integrity. Check Freeze Dryer Working Principle.
How does lyophilization work?
Lyophilization involves freezing the material and then subjecting it to vacuum pressure. This low-pressure environment allows the frozen water to sublimate directly from solid to vapor without passing through a liquid phase, resulting in a dry product.
What are the applications of lyophilization?
Lyophilization is used in various industries, including pharmaceuticals, food preservation, and biotechnology. It is commonly employed to stabilize drugs, vaccines, enzymes, antibodies, and certain food products.
Why is lyophilization used in pharmaceuticals?
In the pharmaceutical industry, lyophilization is used to increase the stability and shelf life of drugs, vaccines, and biologics. It removes moisture from these sensitive materials, preventing degradation and extending their usability. More on Lyophilizer Drug Stability.
What types of materials can be lyophilized?
Lyophilization is suitable for materials that are sensitive to heat or pressure, such as proteins, vaccines, antibiotics, enzymes, and certain food products like fruits and dairy.
What are the benefits of lyophilization?
The benefits of lyophilization include extended shelf life, preservation of product quality, retention of biological activity, and ease of storage and transportation.
Is lyophilization a cost-effective process?
While lyophilization may involve higher initial costs due to equipment and energy requirements, its benefits in terms of product stability and shelf life often outweigh the expenses, especially for sensitive materials and high-value products. Read about Is a Freeze Dryer Worth the Money?
How can I ensure the success of the lyophilizer process?
Success in the lyophilizer process depends on factors such as proper selection of materials, optimization of freeze-drying parameters, validation of process parameters, and adherence to good manufacturing practices (GMP) and quality standards. Working with experienced professionals and conducting thorough testing and validation are essential for achieving desired results. By process optimization, validation, and Lyophilizer Qualification Guidelines.