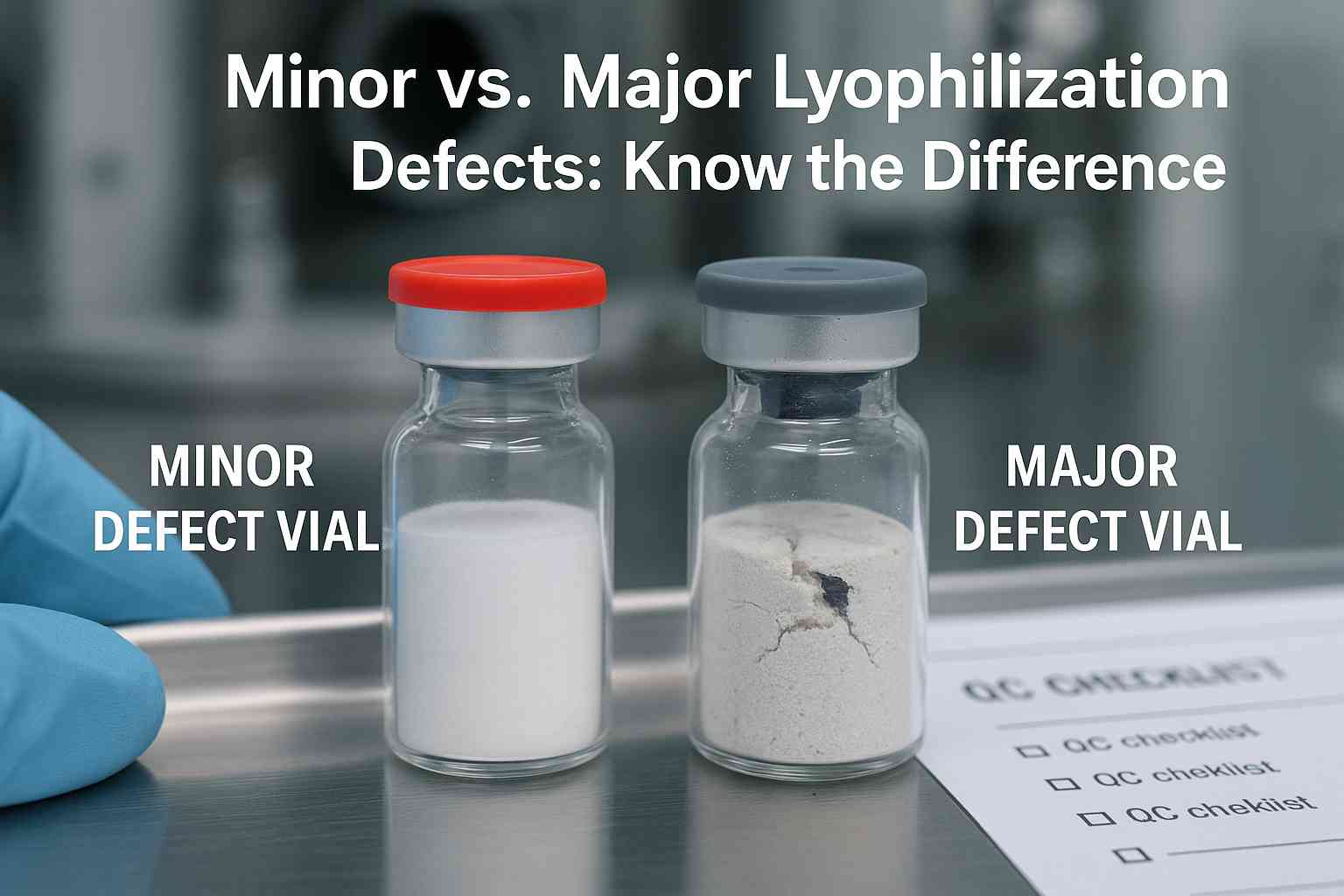
Minor vs. Major Lyophilization Defects are a critical concern in the pharmaceutical industry, especially for injectable drugs, biologics, and vaccines. Lyophilization, or freeze-drying, plays a pivotal role in ensuring the long-term stability and potency of temperature-sensitive substances. However, the integrity of lyophilized products can be compromised by various defects that emerge during or after the lyophilization process.
These defects are typically classified into two categories: minor and major. Understanding the distinction between them is essential for maintaining product quality, ensuring patient safety, and meeting regulatory compliance.
In this article, we’ll explore the differences between minor and major lyophilization defects, how they occur, how to detect and address them, and how manufacturers can prevent them in their freeze-drying processes.
What Are Lyophilization Defects?
Lyophilization defects refer to abnormalities or failures in the freeze-dried product, container, or closure system. These defects can result from improper process parameters, equipment failure, environmental contamination, or formulation instability. Based on their severity and impact on product quality and safety, they are divided into minor and major defects.
Major vs. Minor Lyophilization Defects: Key Differences
| Aspect | Major Defects | Minor Defects |
|---|---|---|
| Impact | Critical: affects safety, efficacy, or sterility | Cosmetic or minimal impact on product performance |
| Examples | Meltback, foreign matter, collapsed cake, chipped glass, high/low dose | Cake chipping, color variation, scratches, improper crimping, seal denting |
| Regulatory Concern | High: may result in batch rejection or recall | Moderate: may require inspection or rework |
| Action Required | Immediate investigation, root cause analysis, possible rejection | Monitoring and corrective measures |
| Detection Method | Visual, automated systems, sterility testing, vacuum/moisture analysis | Visual inspection, physical handling |
Major Lyophilization Defects
Those defects significantly compromise the safety, efficacy, or usability of the product. They often require batch rejection and thorough root-cause analysis.
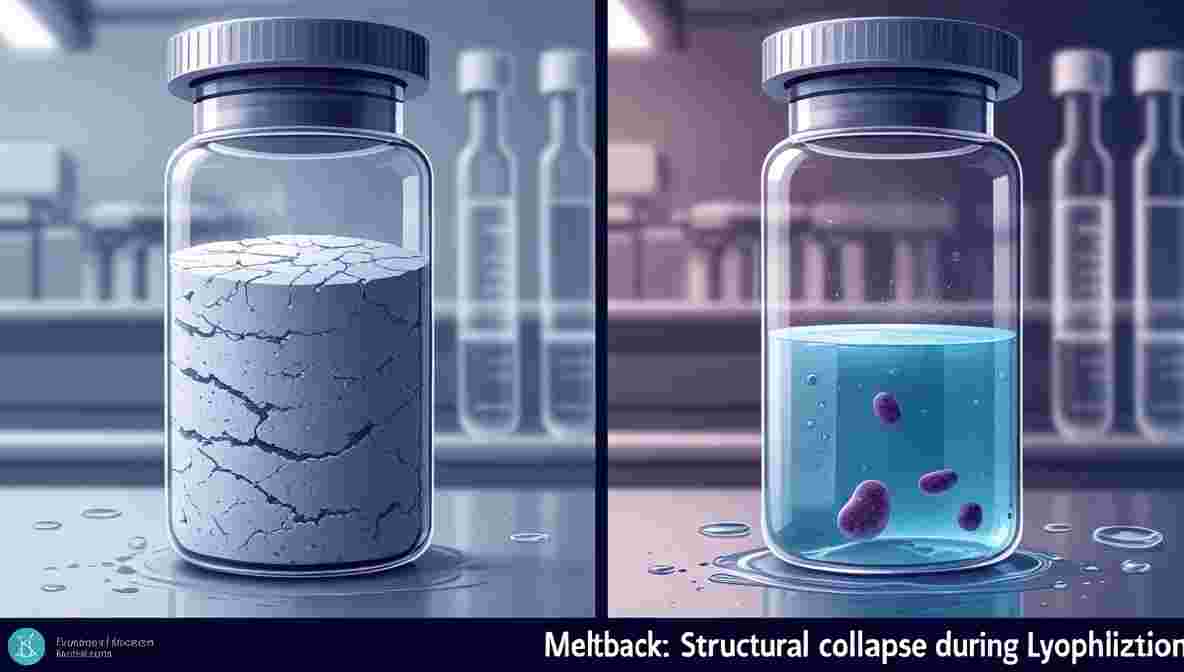
Meltback: Meltback occurs when the lyophilized cake partially or completely liquefies during the process or storage. It results in a loss of structure, compromised stability, and potential microbial contamination.
- Read more about this in our dedicated guide: Meltback Defect in Lyophilized Cake: Key Causes & Solutions
Foreign Matter Contamination: Contaminants like glass shards, rubber particles, or dust can enter the vial and render the product unsafe.
Collapsed Cake: This occurs when the cake structure fails, appearing sunken or liquefied. It indicates inadequate sublimation or improper shelf temperature control.
Vacuum Failure or Inadequate Stoppering: If vacuum levels are not properly maintained or the stopper isn’t applied under vacuum, moisture may enter the vial, compromising product stability.
Glass Particle Contamination: Breakage or delamination of glass vials can introduce particles into the product, a serious regulatory concern.
- Detailed insights here: Glass Particles in Injectable Drugs
Dose Variation: Inconsistencies in the amount of drug substance between vials can directly affect dosing accuracy.
- Understand it better with: Understanding Dose Variation in Freeze-Dried Pharmaceuticals
Minor Lyophilization Defects
Minor defects do not pose immediate risk to product safety or efficacy but may affect aesthetic quality, customer perception, or product shelf life.
Fogging or Vial Staining: Residual product might deposit on the inner vial walls due to vapor condensation during freeze-drying.
Micro-collapse: Slight collapse in the cake structure without compromising moisture content or drug stability. Usually cosmetic.
Chipping of Cake Corners: Caused by vial vibration or handling during transport. It doesn’t affect product efficacy but is undesirable visually.
Color Variation: Differences in color across batches or within a batch may indicate formulation inconsistencies but not necessarily quality degradation.
Surface Roughness: Uneven or granular cake surfaces due to cooling or formulation issues. Generally considered non-critical.
Critical vs. Non-Critical Defects: How They’re Evaluated
Regulatory guidelines and Good Manufacturing Practices (GMP) stress the importance of defect classification in lyophilized products. Refer to this full guide on Defect Classification in Lyophilized Products
Batch release decisions rely on the severity and frequency of defects. A single major defect can result in batch failure, while multiple minor defects may lead to re-inspection. For a full checklist on batch inspection, visit: Freeze Dryer Batch Inspection Checklist
Causes of Minor vs. Major Lyophilization Defects
- Improper Freeze-Drying Cycle Design
- Incorrect Loading or Fill Volume
- Uncalibrated Sensors or Equipment
- Vacuum Leakages: Learn How to Identify Them Freeze-Drying Unit Leak Test
- Improper Cleanroom Practices
- Glass Vial Incompatibility
- Excess Product Residue or Surface Tension Effects
Detection & Inspection Techniques of Minor vs. Major Lyophilization Defects
- Visual Inspection: Detects cake collapse, discoloration, and foreign particles.
- Weight Uniformity Testing: Checks dose consistency.
- X-ray or Automated Systems: For non-visible internal defects.
- Vacuum Testing & Moisture Content Analysis: Ensures closure integrity.
Explore the Pharmaceutical Freeze-Drying QA Guide to strengthen your QA protocols.
Preventing Minor vs. Major Lyophilization Defects: Best Practices
Optimized Cycle Development: Tailor primary and secondary drying phases to the specific formulation. Get started with: Lyophilization Cycle Development Guide
- Robust Equipment Qualification & Calibration
- Regular Preventive Maintenance
- In-Process Monitoring & Alarm Handling: Refer to: Freeze-Drying Alarms & Pressure Deviation Guide
- Proper Cleanroom Design & Hygiene Control: Understand requirements: Lyophilization Room Requirements
- Validated Cleaning Processes: Read more: Lyophilizer Cleaning Validation
Regulatory Compliance for Minor vs. Major Lyophilization Defects Management
Regulators such as the FDA and EMA expect manufacturers to classify and manage product defects using risk-based approaches. Defect acceptance criteria must be scientifically justified and validated. Non-compliance may lead to warning letters or recalls.
Stay compliant with: Regulatory Compliance for Lyophilized Products
Final Thoughts of Minor vs. Major Lyophilization Defects
The distinction between minor and major lyophilization defects is essential to understand for quality control, regulatory compliance, and patient safety. While minor defects may seem harmless, recurring cosmetic flaws can signal deeper systemic issues. Conversely, major defects demand immediate corrective action.
By proactively monitoring your lyophilization process, using the right analytical tools, and following cGMP protocols, you can reduce both minor and major defects, protect product integrity, and ensure successful market release.
For further guidance, don’t miss our complete article on How to Identify Critical Defects in Lyophilized Pharmaceuticals
- Defects in Lyophilized Product – A Complete Easy Guide
- Lyophilized Product Quality Standards
- Applications of Freeze-Drying in Biopharmaceuticals
✅ FAQs on Minor vs. Major Lyophilization Defects
What are lyophilization defects in pharmaceuticals?
Lyophilization defects refer to any abnormalities occurring in freeze-dried drug products, vials, or seals that affect quality, safety, or appearance.
How are lyophilization defects classified?
They are classified into two types—minor and major—based on their impact on product quality, safety, efficacy, and regulatory compliance.
What are considered major lyophilization defects?
Major defects include meltback, foreign matter, glass breakage, incorrect dosing (high/low), and vial contamination—issues that can compromise sterility or efficacy.
What are common minor defects in lyophilized vials?
Minor defects include improper cake appearance, stains, scratches, wavy seals, improper crimping, and flip-off seal issues, which mostly affect appearance.
Can minor lyophilization defects be ignored?
While they may not affect drug safety, recurring minor defects may signal process issues and negatively affect patient trust and regulatory inspections.
How are major lyophilization defects detected?
Through visual inspection, weight checks, vacuum testing, and sometimes automated detection systems like X-ray or spectroscopy.
What causes major defects during freeze-drying?
They often result from equipment failure, poor cycle design, improper sealing, formulation instability, or contamination during processing.
How can minor and major defects be prevented?
By using optimized freeze-drying cycles, regular equipment calibration, cleanroom controls, and robust inspection protocols.
Are all visible issues classified as major defects?
No. Some visible changes, like fogging or minor cake imperfections, may be cosmetic (minor) and not affect product quality.
Do regulatory bodies differentiate between minor and major defects?
Yes. Regulatory guidelines require strict control over major defects and recommend quality systems to monitor and manage minor defects consistently.