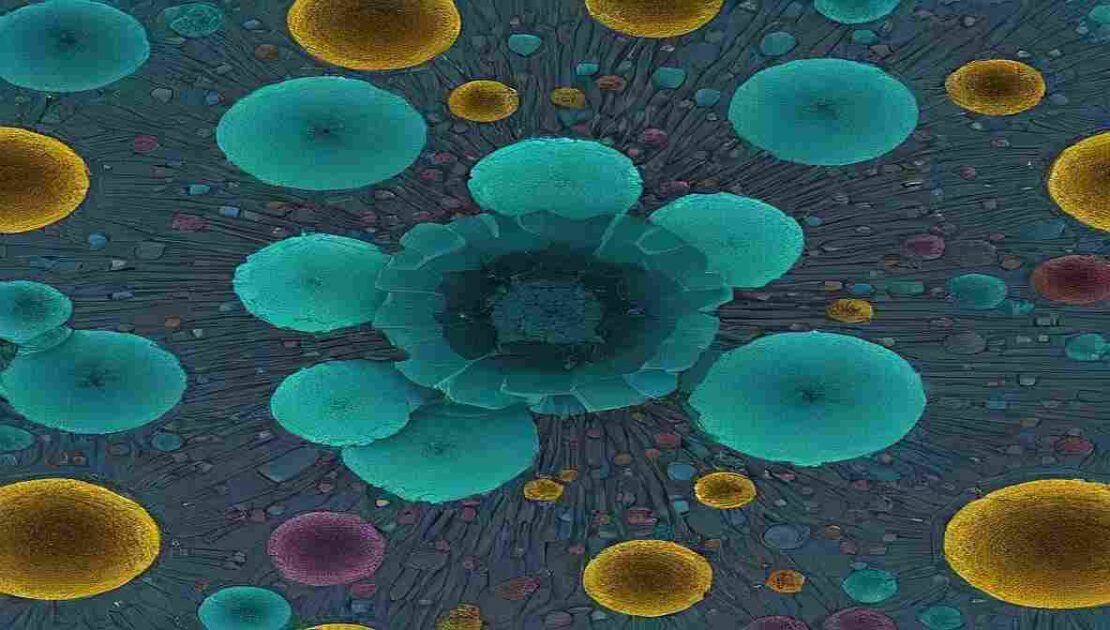
In modern pharmaceutical manufacturing, Cryo-Microscopy in Freeze-Drying has emerged as a powerful analytical tool to visualize the microstructure of lyophilized products.
This technique, particularly cryo-electron microscopy (Cryo-EM), provides high-resolution insights into the internal morphology and porosity of the freeze-dried “cake,” a critical determinant of product stability and reconstitution performance.
Understanding the cake structure at the microscopic level helps researchers optimize formulation design, freeze-drying cycles, and overall product quality — making Cryo-Microscopy a vital part of advanced lyophilization analytics.
The Role of Cryo-Microscopy in Freeze-Drying Process Development
Cryo-Microscopy provides structural clarity that traditional microscopy methods cannot achieve. By maintaining the sample in its frozen state during imaging, Cryo-EM prevents structural deformation and sublimation artifacts.
This preservation allows researchers to capture the true pore morphology and ice crystal distribution, directly linked to heat and mass transfer efficiency during the primary and secondary drying phases.
Through this advanced visualization, scientists can correlate microscopic features with critical process parameters such as shelf temperature, chamber pressure, and sublimation rate — improving reproducibility and batch consistency.
For more insights into optimizing process efficiency and validation, refer to PAT (Process Analytical Technology) in Freeze-Drying.
Understanding Cake Morphology Using Cryo-Electron Microscopy
1. Structural Preservation and Imaging
Cryo-EM preserves the delicate lyophilized matrix by keeping it below the glass transition temperature of the formulation. The sample is transferred under cryogenic conditions, ensuring minimal moisture absorption and no melting of the ice matrix.
This process provides precise visualization of pore networks, interconnected channels, and solid-phase boundaries that define the mechanical strength and reconstitution rate of the final product.
2. Evaluating Ice Crystal Distribution
Cryo-Microscopy in Freeze-Drying enables analysis of ice crystal size, orientation, and distribution, which directly influence sublimation kinetics. Uniform ice crystal growth results in consistent porosity and faster sublimation, while heterogeneous crystal distribution can lead to structural collapse or incomplete drying.
Such detailed cryogenic imaging allows for better formulation engineering and controlled freezing — aligning with smart freeze-drying strategies seen in Lyophilization 4.0 technologies (Read here).
Cryo-EM vs. Conventional Microscopy in Freeze-Drying Studies
While traditional scanning electron microscopy (SEM) provides valuable surface data, it often introduces artifacts due to dehydration and coating.
Cryo-EM, however, maintains native microstructures without distortion, allowing for accurate analysis of the inner cake matrix.
| Parameter | Conventional SEM | Cryo-Electron Microscopy (Cryo-EM) |
|---|---|---|
| Sample Preparation | Requires drying and coating | Maintained under cryogenic conditions |
| Resolution | Moderate | High (near-atomic level for some samples) |
| Artifact Formation | High (due to dehydration) | Minimal |
| Structural Integrity | Altered | Preserved |
| Application | Surface imaging | Internal morphology analysis |
Thus, Cryo-Microscopy in Freeze-Drying is indispensable for real-time understanding of product integrity and microstructural fidelity.
Correlation Between Cake Structure and Product Performance
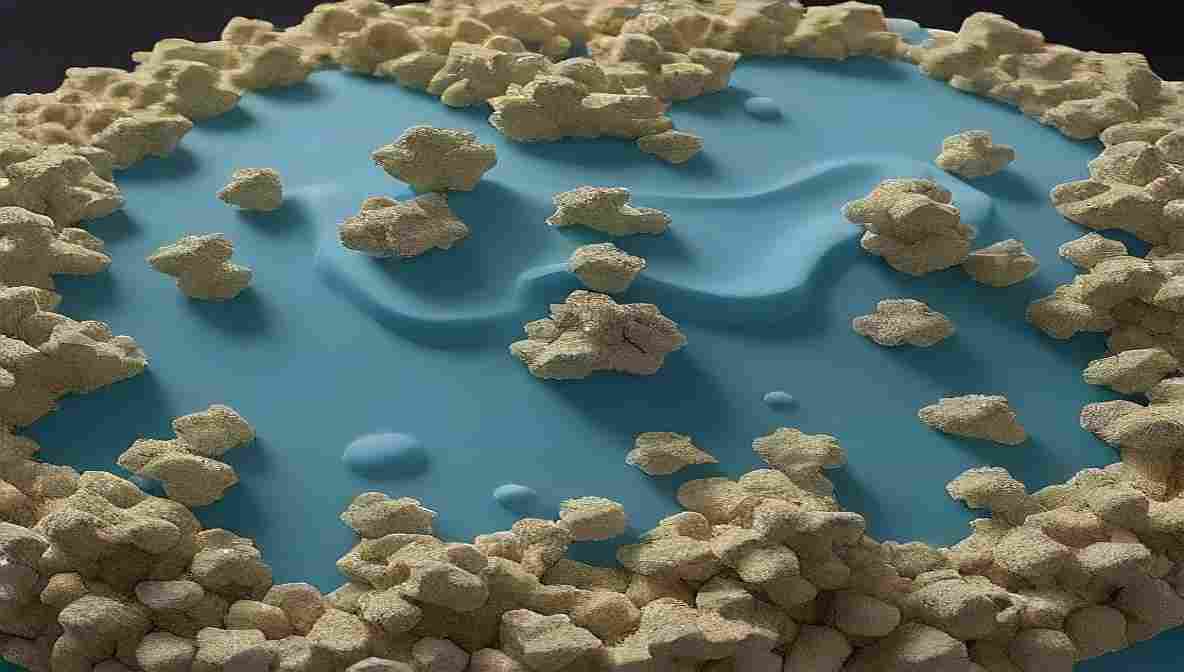
A stable freeze-dried product must have a uniform, porous cake structure that allows rapid rehydration and long-term stability. Cryo-EM helps quantify critical attributes such as:
- Porosity and Pore Size Distribution
- Wall Thickness and Channel Uniformity
- Collapse Patterns and Microfractures
- Residual Moisture Retention Zones
Analyzing these features guides process optimization for both heat-sensitive biologics and small-molecule formulations.
Researchers can precisely adjust freezing rates, annealing steps, and drying parameters to improve product reproducibility.
For a deeper look into quality assurance in lyophilization, see Lyophilizer Cleaning Validation.
Cryo-Microscopy in Biopharmaceutical and Vaccine Development
Biopharmaceuticals such as monoclonal antibodies, peptides, and mRNA vaccines are highly sensitive to drying stress. Cryo-EM facilitates detailed visualization of protein aggregation zones, collapse interfaces, and amorphous crystallization boundaries in these complex formulations.
This insight helps formulators fine-tune excipients, stabilizers, and cryoprotectants to enhance the physical and chemical stability of the lyophilized cake — ensuring therapeutic efficacy post-reconstitution.
Advanced cryogenic imaging also supports the development of next-generation biologics, complementing innovations discussed in Freeze-Drying for Cell and Gene Therapy.
Integration of Cryo-Microscopy Data with Process Analytical Technology (PAT)
Modern lyophilization development integrates Cryo-Microscopy data with real-time PAT systems to enable predictive control. Cryo-EM provides structural validation that complements non-invasive sensors such as:
- Tunable Diode Laser Absorption Spectroscopy (TDLAS)
- Near-Infrared (NIR) Spectroscopy
- Thermocouple and Pressure Sensors
Together, they provide a comprehensive understanding of the drying behavior — from molecular arrangement to bulk structural response.
Such integration is key to achieving data-driven manufacturing, a cornerstone of smart lyophilization systems under Industry 4.0.
Cryo-Microscopy Applications in Failure Detection and Troubleshooting
Structural anomalies in freeze-dried cakes — such as cracks, shrinkage, or phase separation — are often invisible to the naked eye.
Cryo-Microscopy provides the precision needed to detect and interpret these microscopic defects.
Common Issues Identified Through Cryo-EM:
- Micro-collapse due to insufficient annealing
- Heterogeneous pore size from uncontrolled freezing rates
- Channel blockages impacting vapor flow
- Crystallization of buffer components
By correlating these structural irregularities with process conditions, engineers can identify root causes and prevent batch failures, improving yield and quality assurance.
For additional troubleshooting approaches, refer to Failure Detection in Freeze-Drying.
Advancements in Cryo-Microscopy Techniques for Lyophilization
Recent developments have enhanced the imaging accuracy and reproducibility of Cryo-EM in freeze-drying research:
- Cryo-Focused Ion Beam (FIB) Milling: Enables cross-sectional imaging without sample distortion.
- Automated Cryo-Tomography: Captures 3D structural data for comprehensive cake analysis.
- AI-Assisted Image Reconstruction: Improves data interpretation and pattern recognition.
- Cryo-Correlative Light and Electron Microscopy (Cryo-CLEM): Merges molecular mapping with structural imaging.
These advancements pave the way for multi-dimensional lyophilization analytics, transforming process development into a data-driven discipline.
Regulatory Relevance of Cryo-Microscopy in Freeze-Drying
Regulatory bodies like the FDA and EMA emphasize robust analytical validation for lyophilized pharmaceuticals.
Cryo-Microscopy supports compliance by providing direct structural evidence of process consistency, aligning with GMP requirements for visualization and documentation.
It is increasingly referenced in lyophilization qualification protocols, supporting critical documentation for validation batches.
For a detailed understanding of GMP standards, refer to GMP Freeze-Drying Guidelines.
The Future of Cryo-Microscopy in Freeze-Drying Research
The fusion of Cryo-Microscopy with machine learning, AI-based pattern detection, and digital twin modeling will redefine analytical approaches.
Future lyophilization systems will use Cryo-EM data as a validation checkpoint for adaptive cycle control — ensuring optimal drying across diverse formulation matrices.
This approach represents the next stage in Lyophilization 4.0, where imaging intelligence meets process automation.
Conclusion
Cryo-Microscopy in Freeze-Drying bridges the gap between structural understanding and process control.
By visualizing the frozen microstructure in its true state, Cryo-EM empowers researchers to optimize parameters, ensure product stability, and predict performance.
As biopharmaceuticals and complex biologics evolve, this analytical tool will remain indispensable in developing robust, high-quality lyophilized formulations that meet both regulatory and clinical demands.
10 FAQs on Cryo-Microscopy in Freeze-Drying
1. What is Cryo-Microscopy in Freeze-Drying?
It’s an imaging technique using cryo-electron microscopy to visualize frozen lyophilized structures without distortion.
2. How does Cryo-EM preserve the sample structure?
It maintains samples below their glass transition temperature, preventing ice sublimation or deformation.
3. Why is cake structure analysis important in lyophilization?
Cake structure determines product stability, porosity, and reconstitution time.
4. Can Cryo-Microscopy detect formulation defects?
Yes, it identifies micro-collapse, cracks, and heterogeneity in freeze-dried cakes.
5. How does Cryo-EM complement PAT systems?
It validates structural insights derived from non-invasive PAT measurements.
6. What are the limitations of Cryo-EM in lyophilization?
High cost, complex sample handling, and cryogenic maintenance requirements.
7. Is Cryo-Microscopy suitable for biological formulations?
Yes, it’s ideal for proteins, vaccines, and gene therapy products sensitive to heat and dehydration.
8. How does freezing rate affect Cryo-EM observations?
Faster freezing creates smaller pores, while slower freezing forms larger crystals — both visible under Cryo-EM.
9. Can AI improve Cryo-Microscopy analysis?
Yes, AI enhances image reconstruction and pattern recognition for better structural interpretation.
10. What future trends involve Cryo-Microscopy in Freeze-Drying?
Integration with AI, digital twins, and predictive control in Industry 4.0 lyophilization systems.