The meltback defect in lyophilized cake is a significant challenge in pharmaceutical manufacturing, particularly during the freeze-drying (lyophilization) process used for injectable formulations, biologics, and vaccines.
This defect compromises the structural integrity and appearance of the lyophilized product, affecting its quality, stability, and compliance with regulatory standards. Understanding and addressing this issue is essential for maintaining product performance and patient safety.
In this article, we’ll explore what meltback is, its underlying causes, detection techniques, and effective prevention strategies.
Table of Contents
ToggleWhat Is Meltback in Lyophilized Products?
Meltback refers to the partial or complete collapse of the lyophilized cake, usually occurring during or after primary drying. It results in a wet, sticky, or glossy appearance instead of the expected solid, porous structure. This defect is typically caused by improper process parameters or temperature excursions, which result in the product melting or reabsorbing moisture.
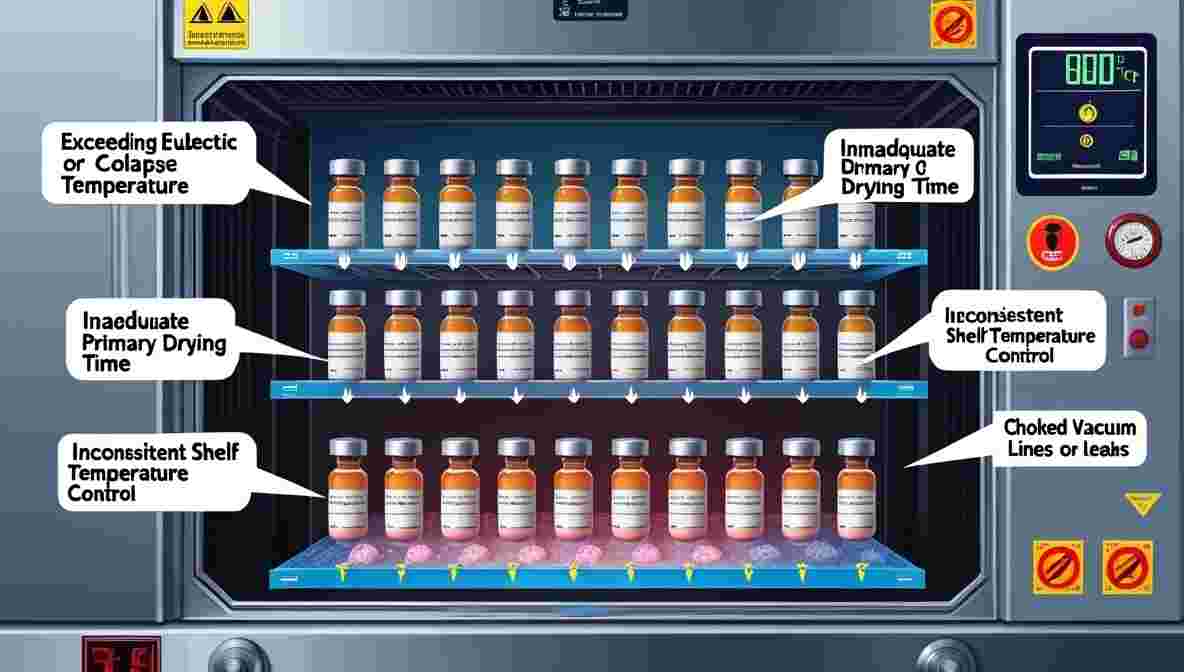
Root Causes of Meltback Defect
Understanding the underlying factors behind the meltback defect in lyophilized cake is key to implementing corrective actions. Common causes include
- Exceeding Eutectic or Collapse Temperature: Operating above the product’s critical temperature leads to structural breakdown.
- Inadequate Primary Drying Time: Insufficient sublimation causes residual moisture, which may recondense during secondary drying.
- Inconsistent Shelf Temperature Control: Non-uniform heating may cause partial melting in certain vials.
- High Product Fill Volume: Excessive liquid volume delays drying and encourages meltback.
- Choked Vacuum Lines or Leaks: These reduce pressure control efficiency and disrupt sublimation.
For an in-depth understanding of how process variables influence drying, you can refer to Freeze Drying Process Parameters: An Essential Guide.
Visual Characteristics of Meltback
A product exhibiting meltback may display:
- Glossy or glassy surface
- Shrinkage or sunken center
- Sticky or semi-solid texture
- Cloudy or hazy vial interior
These visual cues are often detected during batch inspection. A helpful checklist can be found in this Freeze Dryer Batch Inspection Guide.
Risks Associated with Meltback
Meltback is more than a cosmetic flaw—it’s a red flag for quality and safety.
- Loss of Cake Structure: Affects reconstitution time and accuracy.
- Reduced Stability: High residual moisture encourages microbial growth.
- Potency Loss: Thermal or moisture degradation of active ingredients.
- Regulatory Rejection: Fails to meet pharmacopeial standards.
To avoid these consequences, proper process design and equipment calibration are crucial. Pharmaceutical Freeze-Drying QA Guide offers more on implementing quality assurance protocols.
Detection Techniques for Meltback
Early detection of meltback helps prevent entire batch failures. Techniques include:
-
Visual Inspection
Trained inspectors examine vials post-drying for signs of collapse or glossiness. -
Moisture Content Testing
Karl Fischer titration or near-infrared spectroscopy can quantify residual moisture. -
Thermocouple Mapping
Identifies temperature inconsistencies across shelves. -
Vacuum Profiling
Anomalies in pressure readings may hint at incomplete sublimation or meltback onset.
For insights into identifying other critical defects, refer to
👉 How to Identify Critical Defects in Lyophilized Pharmaceuticals
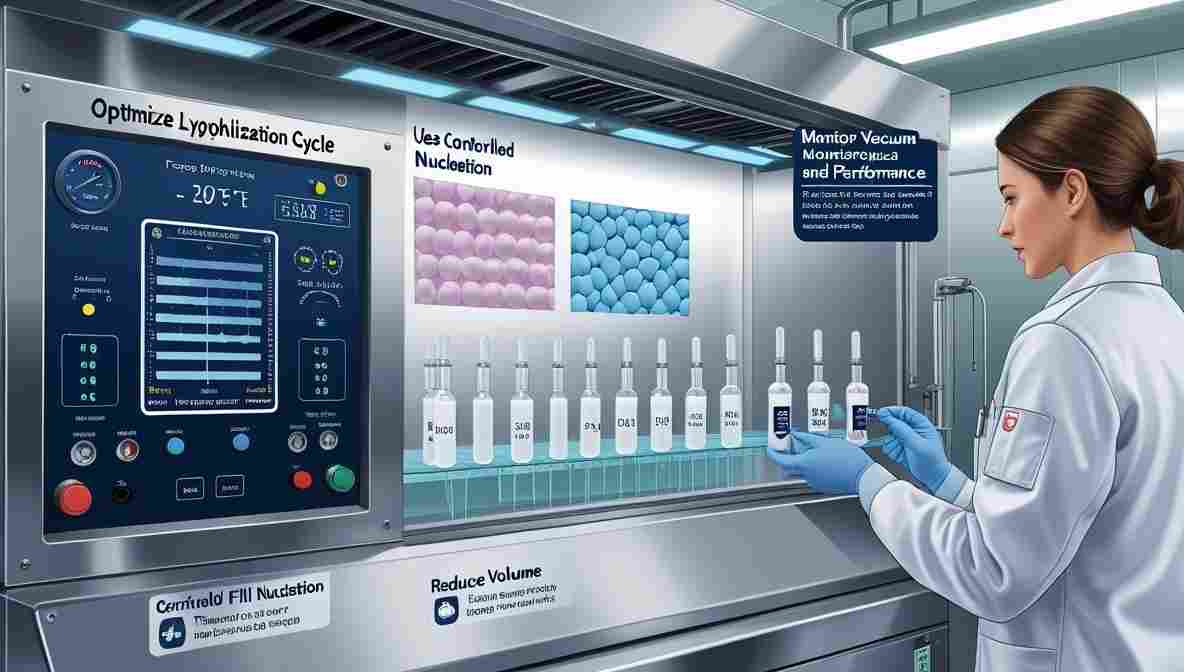
Prevention Strategies of meltback
To minimize the occurrence of meltback, manufacturers can:
- Optimize Lyophilization Cycle: Adjust shelf temperature and pressure to stay below critical temperatures.
- Use Controlled Nucleation: Encourages uniform ice crystal formation, aiding efficient sublimation.
- Reduce Fill Volumes: Ensures even drying and temperature penetration.
- Monitor Vacuum and Shelf Performance: Regular equipment calibration prevents deviations.
Additionally, implementing automated alarms and sensors can alert operators to deviations. For improved design, refer to Energy Efficiency in Lyophilization.
Regulatory and Quality Implications
According to FDA guidelines, products must demonstrate consistency, stability, and freedom from visual and structural defects. Meltback often leads to batch rejection during visual inspection stages. Quality teams must document corrective actions and prevent recurrence through CAPA (Corrective and Preventive Actions) procedures.
You can explore an example of FDA perspectives on lyophilization here:
🔗 FDA’s Pharmaceutical CGMP Guidelines
Related Internal Resource
Understanding foreign matter contamination alongside meltback can improve overall lyophilization quality. Read this detailed guide:
📘 Foreign Matter in Lyophilized Product: A Comprehensive Guide
Conclusion
The meltback defect in lyophilized cake is a preventable but serious quality issue. It arises from poor process control, excessive thermal load, or inadequate drying phases. Through robust quality control systems, regular equipment maintenance, and optimized drying cycles, manufacturers can significantly reduce the risk of meltback and ensure consistent product performance.
For more insights on lyophilization defect classification, visit:
🔎 Defect Classification in Lyophilized Products: A Quality Guide
Suggested Outbound Resources
- FDA on Visual Inspection of Injectable Products
https://www.fda.gov/media/78522/download - ISPE Good Practice Guide: Lyophilization
https://ispe.org/publications/guidance-documents/good-practice-guide-lyophilization
✅ FAQs: Meltback Defect in Lyophilized Cake
Q1. What is a meltback defect in lyophilized cake?
A: Meltback refers to the collapse or partial melting of the lyophilized cake during or after primary drying, often resulting in a sticky, glossy appearance due to improper temperature or vacuum control.
Q2. What causes meltback in lyophilized products?
A: Common causes include exceeding collapse temperature, inadequate primary drying, uneven shelf temperature, and high product fill volume.
Q3. How can meltback be detected during freeze-drying?
A: Visual inspection, moisture testing, thermocouple mapping, and vacuum profiling are standard techniques for detecting meltback defects.
Q4. Why is meltback a concern in pharmaceutical products?
A: Meltback can compromise product stability, sterility, and reconstitution, leading to regulatory non-compliance and potential patient safety risks.
Q5. How can meltback be prevented in freeze-drying?
A: By optimizing the lyophilization cycle, maintaining shelf temperature control, reducing fill volumes, and using proper equipment calibration, meltback can be effectively prevented.