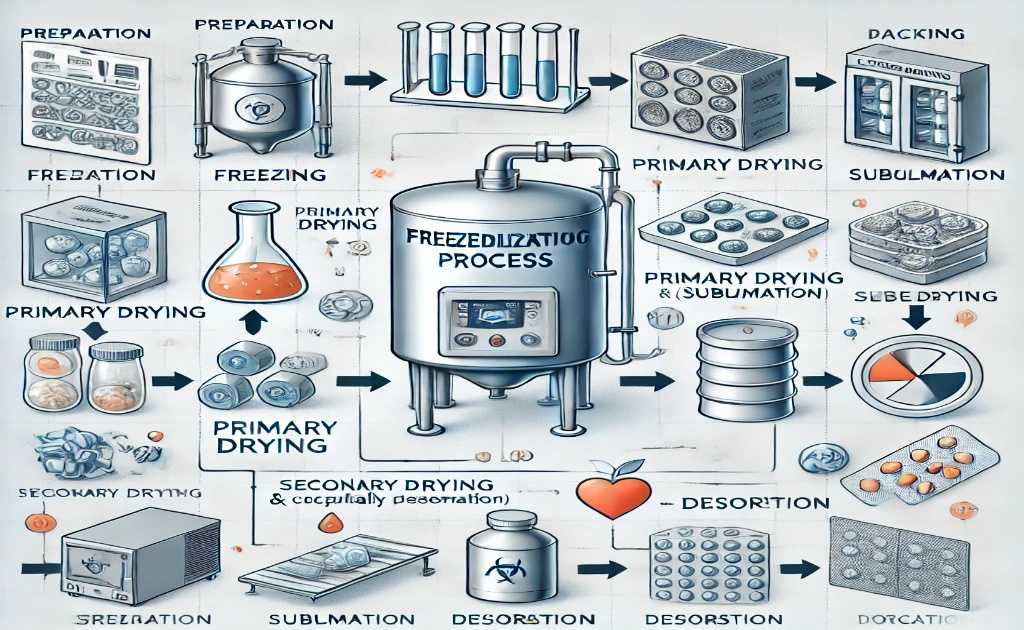
Comprehensive Freeze-Drying Process Flow Chart, We understand the complexities involved in preserving products through freeze drying and have developed a detailed freeze drying process flow chart to guide you through every step.
Our chart captures the essence of lyophilization, a preservation technique essential for products that require stability and longevity, such as pharmaceuticals and various food items. To initiate the freeze-drying process, it is crucial to start with a completely dry system, eliminating any moisture from previous operations.
This ensures optimal performance throughout the intricate stages of lyophilization, detailed meticulously in our lyophilization process flow chart.
The cornerstone of our chart is its ability to demystify the pre-cooling phase, which sets the stage for successful freeze-drying. By illustrating the nuances between a single or dual chamber approach, our chart aids in the decision-making process, effectively underscoring the importance of choosing the right technique for pre-freezing, typically under atmospheric conditions similar to those in a conventional freezer.
Key Takeaways
- Our chart provides an in-depth look at each phase of the freeze-drying process, ensuring you understand the sequence of operations.
- The chart emphasizes the significance of starting with a dry system to guarantee efficiency in lyophilization.
- Understanding the use of pre-cooling to enhance the freeze-drying system’s performance is a key insight offered.
- The delineation between single or dual chamber freezing methods in the chart helps customize the process according to product requirements.
- The freeze-drying process flow chart is a valuable educational tool for those looking to preserve product integrity and extend shelf life through lyophilization.
Understanding the Fundamentals of Freeze Drying
As we embark on the intricacies of the freeze-drying process, also known as lyophilization, it’s essential to comprehend its foundational steps. Our journey begins with a critical phase that sets the stage for successful preservation—the pre-preparation. Following this, mastering the manipulation of device parameters becomes the essence of efficiency and quality in our freeze-drying endeavors.
The Importance of Pre-Preparation
Pre-preparation is an indispensable precursor in the freeze-drying steps. This stage involves careful consideration of the product’s composition and characteristics.
Mastering Device Parameters: Vacuum and Shelf Temperature
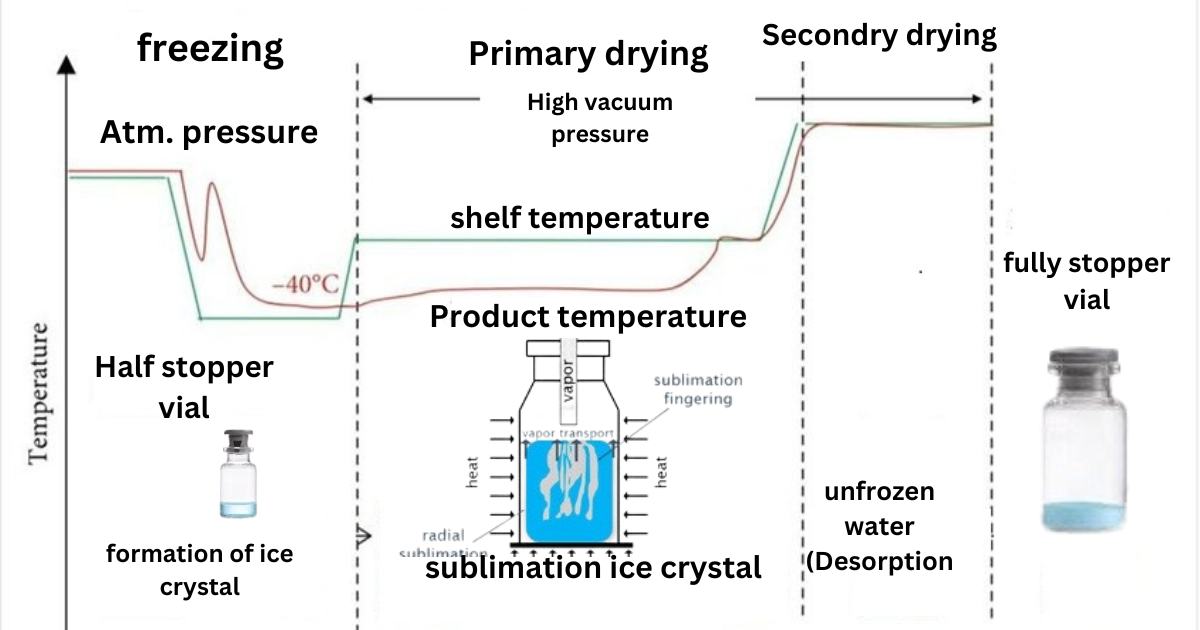
One could say that the heart of lyophilization beats within the precise control of the freezer’s vacuum settings and the shelf temperature. It’s these device parameters that intricately adjust the drying process, aligning it with time-dependent requirements specific to each product. Whether dealing with pharmaceuticals or delicate biological materials, vacuum and temperature management dictate the operation’s pace and outcome. To visually illustrate how these factors come into play, let us explore a freeze-drying diagram
| Parameter | Function | Impact on Freeze Drying |
|---|---|---|
| Vacuum Settings | Controls the pressure within the chamber | Facilitates the sublimation of ice directly from solid to gas |
| Shelf Temperature | Maintains the product temperature | Influences the rate of sublimation and ensures structural integrity |
Through the control and understanding of these steps and diagrams, our goal is to not just document the procedure but to perfect it, constantly pushing the boundaries of what we can preserve and how we can preserve it.
The Pre-Treatment Phase in Freeze Drying
As we delve into the intricate freeze drying process steps, it’s imperative to address the pre-treatment phase, a cornerstone for a successful freeze drying cycle. This initial stage sets the foundation for the quality and efficiency of the entire process. Here, we focus on the meticulous preparations required to achieve the best possible results from freeze-drying.
Preparing the Product for Optimal Results
To kick-start an effective freeze-drying cycle, ensuring the product’s readiness is paramount. We aim for uniformity in size and thickness to facilitate an even and efficient drying process. It’s not just about the end result; it’s about crafting the perfect beginning.
Importance of Freeze Drying System Cleanliness
Cleanliness isn’t merely a virtue; it’s a necessity. The integrity of the freeze-drying process lies in our meticulous nature. By cleansing the system of any residual moisture prior to introducing a new product cycle, we circumvent the risks of partial thawing, ensuring that each cycle commences without the ghosts of the past compromising our results.
Freeze-Drying Process Flow Chart: A Step-by-Step Guide
Before embarking on the complex journey of lyophilization, one must understand the various lyophilization steps involved. Luckily, a lyophilization diagram serves as a visual cue to the process, ensuring no step is overlooked. This systematic approach ensures each phase of the freeze-drying process translates into premium quality and effective preservation.
We often emphasize the critical aspects of pre-treatment, which includes equilibrating the product to ideal conditions before the actual freeze drying begins. Following this, the product undergoes freezing, taking careful note that temperature is meticulously controlled to prevent any damage to its structural integrity.
Lastly, we elevate our attention to secondary drying or the final desorption step. Slight increases in temperature encourage the bound moisture within the product to evaporate, skillfully reducing water content to the lowest possible levels without compromising the product’s quality.
- Pre-treatment: Preparation of the material to optimal conditions.
- Loading Process:The loading phase begins with preparing drug products for lyophilization.
- Freezing: Rapid or controlled freezing to form a solid matrix.
- Primary Drying: Lowering pressure and sublimating the ice directly to vapor.
- Secondary Drying: Elevating temperature to remove bound water.
Steps involved in freeze-drying process flow
Loading stage
After starting the freeze dryer, the loading temperature is achieved, then the freeze dryer door is opened on the aseptic area side, and the empty and filled vial is loaded into the freeze dryer. The freezing cycle is then started by SCADA (Supervisory Control and Data Acquisition) systems, which are used for controlling and monitoring the freeze drying process or and loading
Loading stage: Once the freezer dryer is started and the loading temperature is reached, the door is opened in a sterile area. Empty and filled vials are then loaded into the freeze dryer before initiating the freezing cycle using SCADA.
Loading Process:The loading phase begins with preparing drug products for lyophilization. All depyrogenated, drug-filled vials are carefully loaded into the freeze dryer chamber for the freezing process. Additionally, partially lyophilized vials are reloaded into the freeze dryer to complete the lyophilization process and obtain the final lyophilized powder .
Freezing
A material changes from a liquid to a solid state during freezing.

Primary drying (sublimation)
During the primary drying phase, water vapor adheres to and solidifies on the cold condenser surface, protecting the vacuum pump from moisture. Approximately 95% of the material’s water content is removed during this slow process.

Secondary drying (desorption)
Remaining moisture is removed by further lowering pressure and raising temperature. or Water desorption from the cake starts in the same spot as primary freeze-drying is finished and all ice has been removed via sublimation. The primary drying phase begins when this stage, called secondary drying, begins.

Venting and Stoppering of vials
After completing the secondary drying process, vials are fully stoppered in the freeze dryer using the stoppering force applied by the shelf. venting the chamber with nitrogen 5.0 and stopping the vials inside the freeze dryer in nitrogen atmosphere
Unloading stage
After completion of the freeze drying and stoppering stages, reach the unloading temperature, open the door on the aseptic area side, and unload the freeze drying product.
In our next sections, we will dive deeper into each of these critical phases, offering insights and guidelines on how to navigate the lyophilization landscape and ensure the integrity of the product is maintained throughout the process. This step-by-step guide is a testament to the meticulous nature of lyophilization, highlighting the importance of each phase for achieving the perfect dry state suitable for long-term storage.
Lyophilization Process Steps
| Step | Duration | Temperature | Pressure | Description |
|---|---|---|---|---|
| Loading | 10 min | 10.0 °C | – | Depyrogenated, drug-filled vials are loaded into the freeze dryer chamber for freezing. |
| Freezing | 1 min | 10.0 °C | – | The drug solution is initially frozen to stabilize the product before drying. |
| Primary Drying | 6 min | -40.0 °C | 0.266 mbar | The frozen water is sublimated under vacuum, removing most of the moisture content. |
| Secondary Drying | 180 min | 15.0 °C | 0.066 mbar | Residual water is removed by desorption at a higher temperature to ensure product stability. |
| Stoppering | 1 min | 25.0 °C | 0.066 mbar | The vials are stoppered with the help of shelve pressure under vacuum or inert gas to protect the lyophilized product. |
Explanation of Each Step:
- Loading: The vials containing drug solutions are placed inside the freeze dryer chamber at a controlled temperature of 10.0°C to prepare for freezing.
- Freezing: The temperature is maintained at 10.0°C for 1 minute to begin the freezing process, ensuring uniform solidification of the product.
- Primary Drying: At -40.0°C and a vacuum pressure of 0.266 mbar, the ice in the product undergoes sublimation, turning directly into vapor and being removed.
- Secondary Drying: This phase lasts for 180 minutes at 15.0°C with a lower pressure of 0.066 mbar to remove any remaining moisture and ensure long-term stability.
- Stoppering: The vials are sealed at 25.0°C under a vacuum of 0.066 mbar to prevent contamination and maintain the integrity of the lyophilized drug.
Embarking on the Freezing Process
As we commence the lyophilization cycle, selecting the appropriate freezing methodology is a critical decision that influences the final product’s quality. Understanding the implications of the freezing process helps us to appreciate the subtleties involved in lyophilization and to employ the most suitable techniques.
Single Chamber vs Dual Chamber Method
In the lyophilization cycle, the freezing stage sets the stage for the entire process. Two primary methods, the single chamber and the dual chamber techniques, stand out. Each has its own unique advantages that may better suit different lyophilization needs.
- The Single Chamber Method leverages a streamlined process where both freezing and drying occur in the same chamber. This method is lauded for its simplicity but requires optimal control of the freeze-drying parameters to ensure quality outcomes.
- In contrast, the Dual Chamber Method differentiates the freezing and drying phases into separate chambers. This allows for fine-tuning of conditions suitable for each stage, potentially maximizing the structural integrity and viability of sensitive products.
Effect of Freezing Rate on Drying Efficiency
The rate at which a product is frozen can have profound impacts on the characteristics of the final, lyophilized product. A rapid freezing rate forms small ice crystals, which are preferable for preserving structure and are particularly important for biological samples observed under microscopy.
However, these smaller crystals may also lead to more tortuous pathways for moisture to escape during drying, potentially affecting the efficiency and duration of the lyophilization cycle. Therefore, it’s important to strike a balance based on the specific requirements of the substance being freeze-dried. Here’s a deeper dive into how the freezing rate impacts the lyophilization cycle:
- Preservation of Structure: A fast freezing rate is preferred when structural preservation is paramount. This is especially true for cellular materials, where integrity is directly linked to functionality.
- Drying Efficiency: Our goal is always to optimize the lyophilization cycle for maximum efficiency. Large ice crystals, formed through slow freezing rates, may facilitate quicker primary drying due to larger sublimation channels.
Each step in the lyophilization cycle requires careful consideration of both product nature and end goals. This reflective planning ensures not only the stability of the products but also the preservation of their quality and integrity.
The Primary Drying Stage: Sublimation Dynamics
As we delve into the intricate phases of lyophilization process, we reach the critical primary drying stage. This juncture in freeze drying is pivotal as it involves the fascinating transformation known as sublimation. Here, we witness the direct transition of the frozen solvent from solid to vapor. This phase is not only mesmerizing from a scientific standpoint but also crucial for the quality of the final product.
Role of the Pressure Control Valve
Integral to this stage is the pressure control valve, a component that cannot be understated in its importance. It’s the gatekeeper of the vacuum, dictating the pressure inside the chamber and thereby enabling the meticulous removal of frozen water or solvents as vapor. The precision with which it regulates pressure safeguards the product’s integrity during sublimation.
Understanding the Sublimation Mechanism
Understanding the sublimation mechanism is to appreciate the heart of the lyophilization process. The careful balance of vacuum pressure and heat applied to the frozen product results in a dance of molecules as they skip the liquid phase entirely, leaping directly from solid to gas. This is where the magic happens—it’s a delicate operation, one that underscores the necessity for meticulous control in freeze drying.
| Sublimation Phase | Pressure Control | Heat Application | Duration |
|---|---|---|---|
| Initial | Establishing Base Vacuum | Minimally Applied | Initial Hours |
| Mid-Phase | Pressure Adjustments | Moderately Increased | Mid-Cycle |
| Final | Maintaining Deep Vacuum | Optimized for Sublimation Completion | Completion Hours |
Through each stage of the process, our attentiveness to the foundations of lyophilization, combined with respect for the material we’re preserving, ensures that our products are not merely dried but transformed with care, ready for longevity.
Applying the Secondary Drying Technique
Once we have conclusively navigated through the primary drying phase of the lyophilization cycle, our attention shifts toward the secondary drying technique. This critical juncture of the freeze-drying process’s steps encompasses an intricate balance to ensure the product’s stability for long-term storage.
Navigating the Desorption Step
The desorption step is integral in achieving the meticulous balance required to strip away residual moisture. During this phase, we meticulously synchronize increased shelf temperatures with a deeper vacuum. This dual-action strategy intensifies the removal of bound water molecules, thereby solidifying the product’s longevity.
Optimizing Residual Moisture Levels
A paramount objective during secondary drying is optimizing residual moisture content. Our goal is to minimize moisture to the lowest viable level, a pivotal factor that could enhance or jeopardize the quality of our product.
Maintaining Product Integrity through Process Control
To maintain the utmost integrity of our products, it is imperative we meticulously follow the prescribed lyophilization steps. This allows us to monitor critical parameters such as temperature and pressure and ensure that each stage of the freeze-drying process remains within the ideal range. Diagnostics play a key role here, enabling us to detect and address any deviations swiftly to prevent quality compromise.
Key Considerations for Reliable Equipment Usage
Reliable equipment is the cornerstone of an unerring freeze-drying process. We factor in sensor design, ensuring accurate measurements and data integrity. Compliance with FDA regulations remains a non-negotiable aspect of our operations, while regular online process monitoring allows for real-time adjustments. Below is a table reflecting the intertwining relationship between equipment reliability and process effectiveness in freeze drying.
| Process Phase | Sensor Type | Expected Function | Regulatory Compliance | Online Monitoring |
|---|---|---|---|---|
| Pre-treatment | Mass Flow Sensors | Assure balanced load distribution | 21 CFR Part 11 | Yes |
| Freezing | Thermocouples | Measure product core temperatures | cGMP Standards | Yes |
| Primary Drying | Vacuum Pressure Gauges | Monitor chamber pressure levels | ISO 9001:2015 | Yes |
| Secondary Drying | Capacitance Manometers | Control residual moisture content | EudraLex, Volume 4 | Yes |
Key Points: Capacitance Manometer Physics
General Overview
- Function: Electro-mechanical gauges for measuring both pressure and vacuum.
- Mechanism: Translates diaphragm movement into an electrical signal proportional to pressure.
- Key Components:
- Diaphragm: Exposed to the measured pressure/vacuum.
- Electrode: Ceramic disk with conductive pathways in the reference cavity behind the diaphragm.
- On-board electronics: Amplifies the electrical signal and outputs it for pressure indicators and controllers.
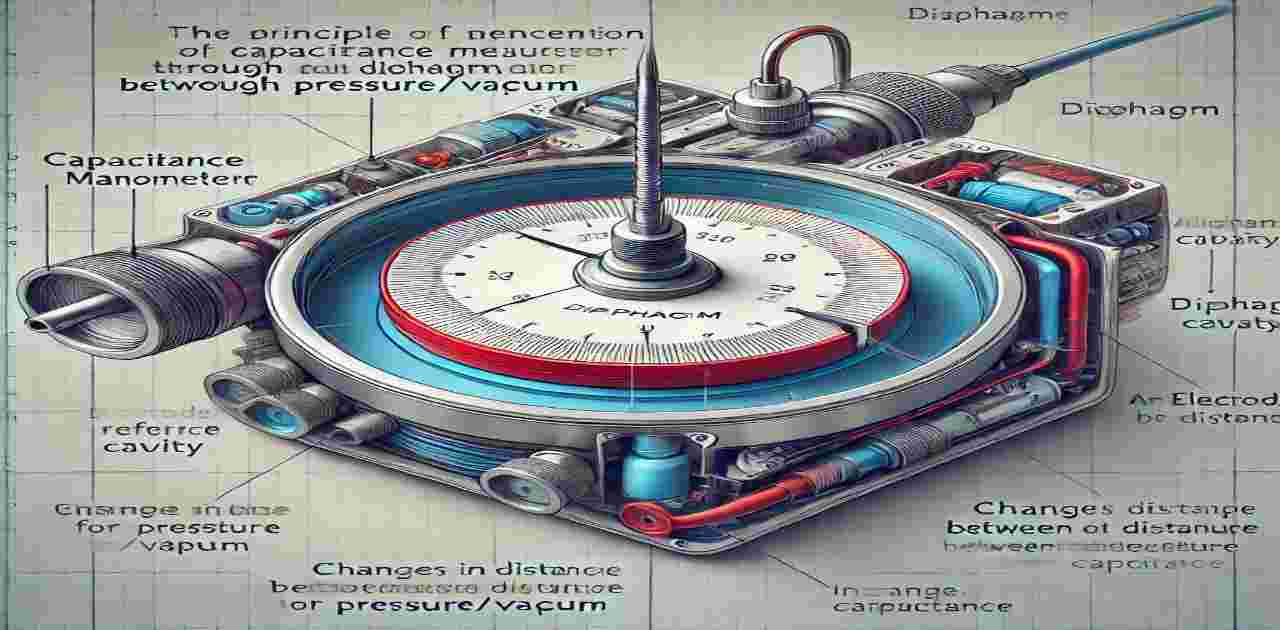
Operating Principle
- Pressure differences deflect the diaphragm, altering the distance between it and the electrode.
- Changes in capacitance (due to diaphragm movement) generate an electrical signal proportional to the pressure.
- Pressure measurement is independent of gas composition, critical for processes with changing chemistry.
Measurement Capabilities
- Operates over a wide pressure range: 1000 Torr to 0.01 Torr.
- Highly accurate: 0.25% of reading, compared to 5-25% for Pirani or thermocouple gauges.
Types of Capacitance Manometers
- Absolute Capacitance Manometers:
- Reference cavity is evacuated to a high vacuum.
- Measurements referenced to vacuum.
- Differential Capacitance Manometers:
- No reference cavity; measures pressure difference between inlet tube and reference cavity.
- Used for safety switches and airflow pressure drop measurements.
Applications
- Reference Standards: Calibration benchmark for other gauge types.
- Semiconductor Industry:
- Absolute manometers monitor in-process pressures due to insensitivity to gas composition.
- Differential manometers are used for pressure-based switching and control in load locks.
Important Notes
- Thermal/mechanical gauges should never be used to calibrate capacitance manometers due to their higher accuracy.
- Workhorse in industries requiring precision vacuum measurements.
Choosing the Right Equipment for Freeze Drying
When it comes to optimizing the freeze-drying steps for your application, the decision on the right equipment is paramount. Not only does it dictate the efficiency of the entire process, but it also influences the quality of the final product. We understand that the journey from a liquid solution to a stable dried form involves a multitude of precisely controlled sublimation stages, all of which are clearly outlined in a freeze-drying process flow chart.
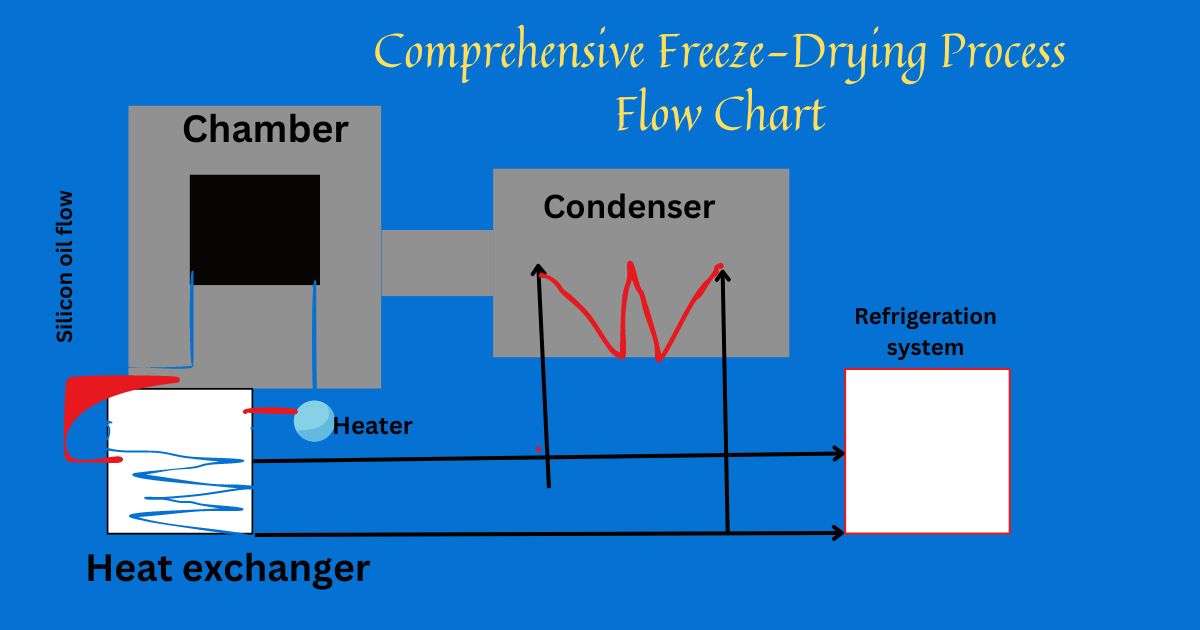
The Impact of Chamber Design on Process Efficiency
Chamber design is the heart of freeze drying systems, affecting everything from drying kinetics to scale-up capabilities.
Vial System Advantages for Complex Products
Employing a vial system within your freeze dryer can offer exceptional benefits, especially when dealing with complex or sensitive products. The vial system provides a controlled environment that can be crucial for maintaining product stability, enabling aseptic processing, and facilitating ease of distribution. Our experience tells us that the precise control over the freeze-drying parameters offered by dedicated vial systems is indispensable for high-value pharmaceuticals or biotechnology products, which demand the highest standards for resale and application.
Mapping the Parameters: Temperature and Pressure in Lyophilization
As we continue our exploration of the freeze-drying cycle,. It is important for us to note the important roles that temperature and pressure play within the lyophilization process. By mapping out the freeze-drying diagram and understanding these parameters, we gain valuable insights that empower us to control the process with precision.
Product and Condenser Temperature Correlation
The careful balance between product and condenser temperatures is at the heart of a successful freeze-drying process. Maintaining optimal temperatures ensures not only the stability of our samples but also the efficiency at which the sublimation phase can proceed. Let’s take a closer look at how these variables interact and why their harmonization is critical.
Approaching the Conclusion of the Freeze-Drying Process
As we reach the final stages of the lyophilization process flow chart,. Our attention shifts toward the safe and effective conclusion of the cycle. Ensuring the sterility and integrity of our products is paramount; therefore, we meticulously follow the established steps for venting the system and removing the dried product.
Procedural Steps for System Venting and Product Removal
Ventilating the system is a delicate process that we perform with the utmost care to prevent any possibility of contamination. Depending on our specific needs, we may introduce ambient air or an inert gas such as nitrogen. This step is not only about preserving the quality of the product at hand. But it is also about preparing the equipment for subsequent use.
Once the environment within the chamber is safely normalized,. The product can be securely removed. This marks an important point. Where the product is ultimately contained is the lyophilization equipment. And transitioned to the next stage of packaging or storage under controlled conditions. To illustrate the importance of this process, let us look at a detailed table that outlines the essential considerations during venting removal
Following this table as a guide, our next steps ensure that the quality of our product remains uncompromised from lyophilization chamber to the end-user. The freeze dryer is then ready for its next cycle, highlighting the cyclical and ongoing nature of the lyophilization process.
Table of Guides: our next steps
| Stage | Action | Purpose | Considerations |
|---|---|---|---|
| Venting | Introduce gas or air to normalize pressure | Prevent product contamination and ensure structural integrity | Selection of inert gas, monitoring of pressure levels |
| Product Removal | Secure removal of the dried product from the chamber | Facilitate transition to packaging or storage without quality loss | Minimizing exposure to ambient conditions and proper handling techniques |
| System Reset | Thawing of the ice condenser | Prepare the freeze-drying system for the next cycle | Ensuring complete removal of moisture, system checks, and diagnostics |
Conclusion of Freeze Drying Process Flow Chart
As we reach the final stage of our detailed exploration of the freeze-drying process. It becomes clear that the practice of lyophilization is a synthesis of art and science. By following the structured approach seen through our lyophilization diagram, those in the pharmaceutical, culinary and other sectors can protect the integrity and longevity of their products. Throughout our journey, we’ve unpacked the subtle layers of preparation, execution, and control that collectively define the lyophilization process.
The Takeaway: Maximizing Efficiency and Quality in Freeze Drying
Our deep dive into lyophilization steps has been more than just procedural; it’s a blueprint for achieving excellence. Emphasizing precision in each phase, from pre-treatment to secondary drying, and cognizance of the minute details. We enable ourselves to extract maximum efficiency and uphold the highest quality standards. Lyophilization may be complex, but with our comprehensive guidance,. We equip practitioners with the expertise to elevate their freeze-drying endeavors to new heights.
Intricacies such as the balancing act of temperature versus pressure. The nuances of ideal equipment selection and continuous monitoring for validation purposes are no longer daunting. They are the stepping stones to mastery and an invitation to turn knowledge into action for exceptional lyophilization outcomes.
FAQ of Freeze-Drying Process Flow Chart
What is the role of the pressure control valve during the primary drying stage?
The pressure control valve is responsible for establishing a vacuum in the freeze-drying system. Allowing the sublimation of frozen water or solvents from the product. It is pivotal for controlling the drying rate and ensuring efficient sublimation.
How does chamber design impact the efficiency of the freeze-drying process?
Chamber design affects the distribution of temperature and vacuum within the system. Influencing the drying efficiency and consistency of the end product. A good chamber design ensures an even freeze-drying process and optimizes thermal transfer.
How do you establish an effective vapor pressure differential during lyophilization?
Establishing an effective vapor pressure differential involves carefully balancing product and condenser temperatures to maximize vapor pressure. Which facilitates the sublimation and removal of ice from the product to the condenser.