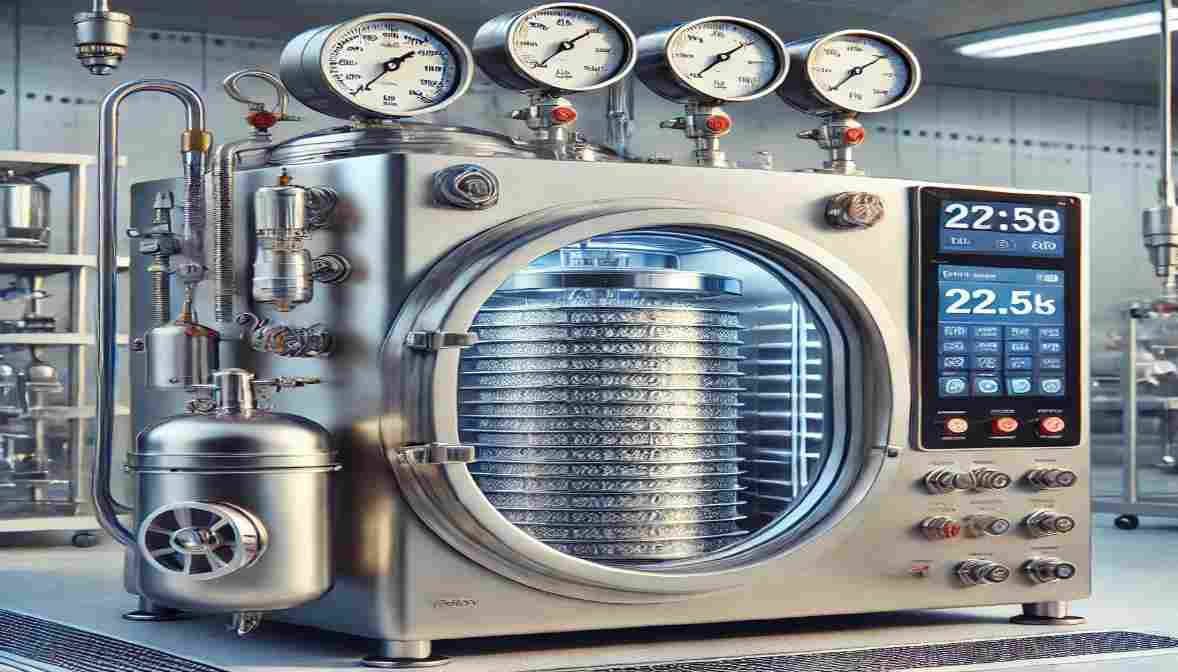
Vacuum Chamber Lyophilization, commonly known as freeze-drying, is a critical process in the pharmaceutical, food, and biotechnology industries. It involves the removal of moisture from products by freezing them and then sublimating the ice under a vacuum. The vacuum chamber plays a crucial role in this process, ensuring controlled environmental conditions to achieve optimal drying efficiency and product stability.
At the heart of this technology is the vacuum chamber, which creates and maintains the precise low-pressure environment required for efficient sublimation. Without accurate vacuum control, freeze-drying cycles can become inefficient, leading to product degradation, extended drying times, or batch failure. Therefore, understanding the function and importance of the vacuum chamber is essential for achieving reliable and high-quality lyophilization results.
The Role of the Vacuum Chamber in Lyophilization.

The vacuum chamber in a freeze dryer is responsible for maintaining the necessary low-pressure environment that facilitates sublimation. By reducing pressure, the boiling point of water decreases, allowing ice to transition directly to vapor without passing through the liquid phase. This helps in preserving the structural integrity, potency, and shelf life of sensitive products
A high-tech vacuum chamber for lyophilization is designed with robust sealing, polished internal surfaces, and precise pressure control systems. These features ensure consistent sublimation rates, uniform heat transfer, and contamination-free processing. The chamber must also withstand repeated thermal and pressure cycles without compromising integrity.
Key Phases of Lyophilization in a Vacuum Chamber
- Freezing Phase: The product is cooled to a temperature below its eutectic or glass transition point. Proper freezing is crucial to avoid issues like temperature overshoots during lyophilization, which can impact product quality.
- Primary Drying (Sublimation) Phase: Under reduced pressure, ice sublimates, leaving behind a porous structure. The vacuum chamber must be tightly sealed and undergo periodic vacuum performance verification to ensure efficiency.
- Secondary Drying Phase: Residual moisture is removed by applying controlled heat, ensuring the final product meets the required stability standards.
Importance of Vacuum Chamber Performance Verification
Regular freeze dryer performance verification is essential to detect potential failures, such as refrigerant compressor issues or vacuum leaks. Any deviation in pressure regulation can compromise drying efficiency, leading to prolonged cycles or incomplete moisture removal.
Common Challenges in Vacuum Chamber Lyophilization
- Vacuum Leaks: A compromised vacuum can lead to inefficiencies. Conducting a freeze-drying unit leak test helps identify potential breaches.
- Compressor Failures: Malfunctioning compressors can lead to excess temperature issues, affecting the chamber’s ability to maintain optimal pressure.
- Inconsistent shelf temperatures: Uneven heating and cooling rates can impact product uniformity. Regular shelf heating and cooling rate verification is crucial.
Enhancing Efficiency in Vacuum Chamber Lyophilization
To optimize lyophilization performance, industries are implementing new improvements in lyophilization services. These include:
- Advanced vacuum monitoring systems for real-time pressure regulation.
- Enhanced automation to reduce human intervention and errors.
- Integration of smart sensors for early failure detection and prevention.
Conclusion
Vacuum chamber lyophilization is a precise and controlled process essential for producing high-quality freeze-dried products. Ensuring proper vacuum conditions through regular lyophilizer cleaning validation and freeze dryer troubleshooting can prevent operational failures and enhance efficiency. By implementing best practices, manufacturers can achieve superior product stability and consistency in freeze-drying applications.
Frequently Asked Questions (FAQs)
What is the role of a vacuum in lyophilization?
The vacuum in lyophilization lowers the pressure within the freeze-drying chamber, allowing ice to sublimate directly into vapor without passing through the liquid phase. This preserves product structure and extends shelf life.
Can you use a vacuum chamber for freeze-drying?
Yes, a vacuum chamber is a fundamental component of freeze-drying. It helps maintain the low-pressure environment needed for effective sublimation and moisture removal.
What is the process of a vacuum chamber?
A vacuum chamber operates by creating a low-pressure environment to facilitate controlled sublimation. In freeze-drying, it enables ice to transition directly to vapor, ensuring efficient moisture removal while preserving product integrity.
What is the difference between lyophilization and vacuum drying?
Lyophilization (freeze-drying) involves freezing a product and then sublimating the ice under vacuum conditions, preserving its structure. Vacuum drying, on the other hand, removes moisture from a product at low pressure without freezing, often resulting in a different texture and stability outcome.
Why is vacuum pressure control important in lyophilization?
Precise vacuum pressure control ensures efficient sublimation during primary drying. Incorrect pressure levels can cause product melting, collapse, or incomplete moisture removal, negatively affecting product quality and stability.
What happens if there is a vacuum leak during freeze-drying?
A vacuum leak increases chamber pressure, reducing sublimation efficiency. This can lead to longer drying cycles, uneven drying, higher residual moisture, and potential batch failure if not detected early.
How is vacuum chamber performance verified in a freeze dryer?
Vacuum chamber performance is verified through pressure rise tests, leak rate testing, vacuum pump efficiency checks, and system integrity validation to ensure consistent low-pressure conditions during operation.
What materials are used to manufacture vacuum chambers for lyophilization?
Vacuum chambers are typically made from high-grade stainless steel, such as SS 316L, which provides excellent corrosion resistance, cleanability, and compliance with pharmaceutical and food-grade standards.
How does shelf temperature uniformity affect vacuum chamber lyophilization?
Uniform shelf temperature ensures even heat transfer across all product containers. Inconsistent temperatures can cause non-uniform drying, leading to variable product quality within the same batch.
What is vacuum capacitor lyophilization?
Vacuum capacitor lyophilization is an advanced freeze-drying approach that uses vacuum-stabilizing components (capacitor-based control systems) to maintain precise and consistent chamber pressure, improving sublimation efficiency, process stability, and product quality during lyophilization.