Residual moisture determination is a critical parameter in the freeze-drying process, directly affecting the stability, shelf life, and quality of the final product. The Karl Fischer method is widely recognized for its precision in measuring water content in solids and liquids.
This method accurately measures both free (adsorbed) and bound (crystalline) water in a sample using Karl Fischer titration, a chemical technique that utilizes methanol as the solvent. It is widely used in the pharmaceutical and freeze-drying industries due to its high sensitivity and precision in detecting trace moisture levels.
The following article explores the details of moisture analysis using Karl Fischer titration, showcasing results from a freeze-drying experiment conducted before and after rehydration. It emphasizes the critical role of accurate residual moisture measurement in optimizing lyophilization cycles and ensuring product stability. For a deeper understanding of the entire freeze-drying process, explore our freeze-drying process and stoppering system.
What is Residual Water Content in Lyophilized Products?
Residual water content refers to the small amount of water that remains in a substance—such as a lyophilized (freeze-dried) powder—even after the freeze-drying process is complete. This leftover moisture is not freely available; instead, it is tightly bound within the dry matrix of the powder. Because of this strong binding, the residual water is very difficult to remove completely, even under extended drying conditions.
During lyophilization, most of the water in a product is removed by sublimation (ice turning directly into vapor) and desorption (removing bound water). However, not all water can be removed. A tiny fraction remains trapped in the structure of the dry powder, and this is called residual moisture or residual water content.
Why Is It Difficult to Remove?
This remaining water is tightly bound at a molecular level within the product matrix. It is held under high tension and doesn’t easily evaporate, even under vacuum and low temperatures. That’s why this moisture is very difficult to eliminate completely.
Why Does Residual Water Matter?
Residual water content is a critical quality parameter in lyophilized pharmaceutical and biotechnological products. Here’s why:
Stability: Too much water can lead to chemical degradation or promote microbial growth.
Shelf Life: Proper residual moisture levels help maintain product effectiveness over time.
Reconstitution: A well-controlled water level ensures smooth re-dissolving when the product is rehydrated.
Storage Conditions: Products with higher residual water may require refrigeration.
How is residual water measured?
Some common methods used in the industry include
Karl Fischer Titration: A precise and widely accepted method for measuring water content.
Thermogravimetric Analysis (TGA): Measures weight loss as the sample is heated.
Near-Infrared Spectroscopy (NIR): A non-destructive method used for moisture analysis.
Residual water content may seem like a small detail, but it plays a vital role in the safety, stability, and performance of lyophilized products. Understanding and controlling this moisture is essential in pharmaceutical manufacturing and quality assurance.
Sampling location for collection of sample after freeze-drying process
After the freeze-drying process is complete, sample collection is carried out at designated sampling points within or immediately outside the lyophilizer chamber. This sampling is done to verify the uniformity, residual moisture, and overall quality of the lyophilized product. Proper sampling location ensures representative results and helps maintain process validation and product compliance.
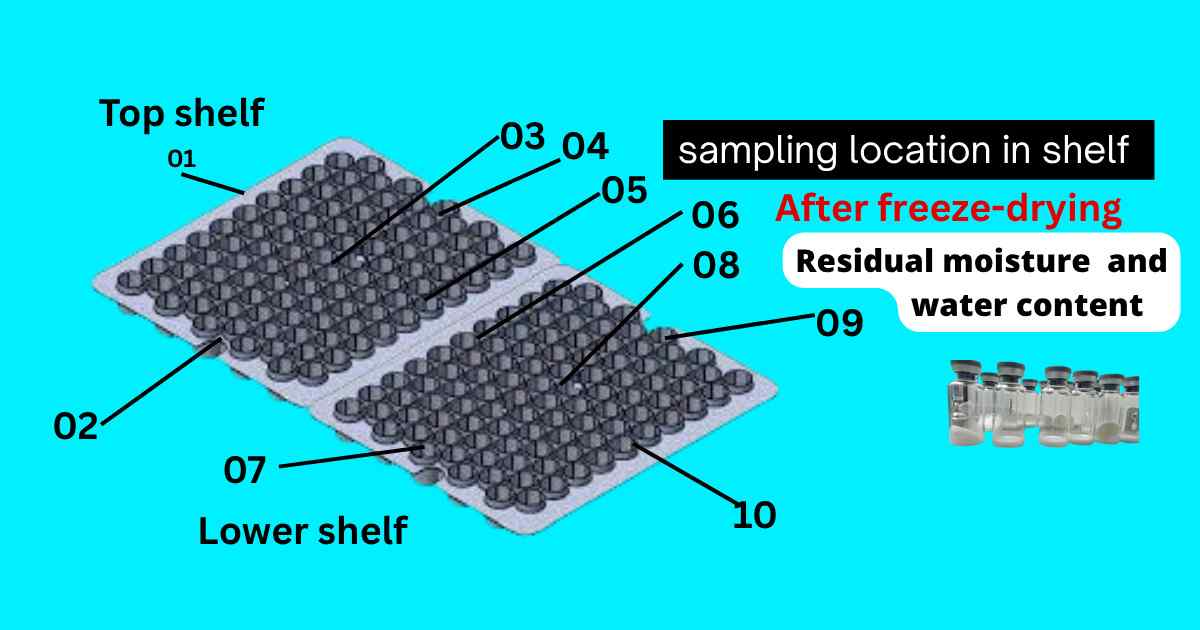
The image shows the identified sampling points across the top and lower shelves of a lyophilizer, used to collect product vials after the freeze-drying process. These locations (marked 01 to 10) represent various positions—corners, center, and edges—on both shelves to ensure uniform drying and accurate analysis.
Sampling from these points helps in:
- Measuring residual moisture and water content
- Ensuring drying consistency across the batch
- Detecting potential cold spots or under-dried areas
This strategic sampling is crucial for quality control, batch release decisions, and process validation in pharmaceutical lyophilization.
Determining the residual moisture after freeze-drying
Determining Residual Moisture Following the Karl Fischer Method The water in liquids and solids can be determined by means of a chemical reaction. Both free (adsorbent water) and bound (crystal water) can be determined. Methanol is used as a solvent in the Karl Fischer reagent (HYDRANAL Composite 5).
The duration of the titration is in general approx. 1-2 minutes. The indication of the terminator point takes place at two electrodes of platinum.
Results of Residual Moisture (Karl Fischer Method) Test

- Karl-Fischer-Titrator
- Solvent: Hydranal-solvent
- Indicator: Hydranal Titrant 5
Summary: Karl Fischer Titrator is an instrument used to accurately measure the residual water content in samples. It uses a special solvent called Hydranal-Solvent and an indicator reagent known as Hydranal Titrant 5 to detect and quantify the moisture present.
Before rehydration: The process was stopped after secondary drying
- Sample weight 0.09790 g, Moisture content 0.35% of Upper right vial
- Sample weight: 0.04078 g Moisture content: 0.68% Upper front vial
- Sample weight: 0.05207 g Moisture: 0.59% Upper left vial
- Sample weight 0.02988 g Moisture: 0.57% Upper rear vial
- Sample weight: 0.09786 g Moisture content: 0.37% Upper middle vial
- Sample weight: 0.05887 g Moisture content: 0.39% Lower right vial
- Sample weight: 0.04718 g Moisture: 0.67% Lower front vial
- Sample weight: 0.06781 g Moisture content: 0.43% Lower left vial
- Sample weight 0.02548 g, moisture content 0.58% Lower rear vial
- Sample weight: 0.04084 g Moisture content: 0.37% Lower middle vial
After rehydration, the process was restarted in the secondary drying step.
- sample weight: 0.02825 g, Moisture content: 4.70%, Upper right vial
- sample weight 0.05203 g Moisture content: 5.01%, Upper front vial
- Sample weight 0.03812 g, moisture content 5.09%, Upper left vial
- sample weight 0.12216 g Moisture content: 4.83%, upper rear vial
- sample weight 0.06297 g Moisture content: 4.90%, upper middle vial
- 6 sample weight 0.03377 Moisture content: 5.34%, lower right vial
- sample weight 0.02687 g Moisture content: 5.22%, Lower front vial
- sample weight 0.09491 g Moisture content: 4.88%, Lower left via
- sample weight 0.06065 g Moisture content: 5.06%, Lower rear vial
- sample weight 0.06420 g Moisture content: 4.85%, Lower middle vial
Freeze-Dried Product Acceptance Criteria
Appearance Test: The product should appear as a white lyophilized cake or powder sealed in clear, colorless glass vials with no visible defects.
Water Content Test: Measured using Karl Fischer coulometric titration, the residual moisture must not be more than 3.0% w/w, ensuring product stability and shelf life.
Residual Solvent Test: Performed using gas chromatography (GC), the tert-butanol (TBA) content must be NMT 4999 ppm (maximum 5000 ppm), meeting regulatory and safety limits.
Conclusion of Residual Moisture Determination
Accurate determination of residual moisture using the Karl Fischer method provides valuable insights into the efficiency of the freeze-drying process. The data from both pre-rehydration and post-rehydration phases emphasize the necessity of meticulous secondary drying to minimize moisture content and ensure product stability. By adhering to precise testing protocols, manufacturers can achieve consistent results, improve product quality, and maintain compliance with industry standards. The Karl Fischer method remains a cornerstone in the analytical toolkit for freeze-drying quality assurance.
Summary of Residual Moisture Determination
The Karl Fischer method effectively measures residual moisture in freeze-dried products, assessing both free and bound water. This chemical titration technique uses methanol as a solvent, delivering rapid and precise results. Data from an experiment conducted before and after rehydration demonstrate the critical role of secondary drying in minimizing moisture content. Accurate residual moisture determination ensures optimal freeze-drying outcomes, enhancing product quality and stability.
FAQs of Residual Moisture Determination
What is the Karl Fischer method used for in freeze-drying?
The Karl Fischer method is used to determine residual moisture content in freeze-dried products by measuring both free and bound water through chemical titration.
Why is residual moisture important in freeze-drying?
Residual moisture affects the stability, shelf life, and quality of freeze-dried products. Minimizing residual moisture is essential to ensuring the product’s long-term viability.
What solvent is used in the Karl Fischer method?
Methanol is used as the solvent in the Karl Fischer method, specifically in the HYDRANAL Composite 5 reagent.
How long does the Karl Fischer titration take?
The titration process generally takes approximately 1-2 minutes.
What were the key findings of the moisture analysis in the experiment?
Before rehydration, residual moisture levels ranged between 0.35% and 0.68%. After rehydration, moisture levels significantly increased, ranging from 4.70% to 5.34%, highlighting the importance of secondary drying in reducing moisture.
What is the role of secondary drying in freeze-drying?
Secondary drying removes bound water from the product, reducing residual moisture content and ensuring better product stability.
Can the Karl Fischer method measure moisture in both liquids and solids?
Yes, the Karl Fischer method is suitable for measuring moisture in both liquid and solid samples.
How does accurate moisture determination benefit the freeze-drying process?
Accurate moisture determination ensures process optimization, product consistency, and compliance with industry standards, enhancing overall product quality.
